Recurrence of COVID-19 associated with reduced T-cell responses in a monozygotic twin pair
- PMID: 35104433
- PMCID: PMC8807054
- DOI: 10.1098/rsob.210240
Recurrence of COVID-19 associated with reduced T-cell responses in a monozygotic twin pair
Abstract
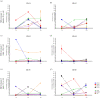
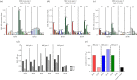
Recurrence of COVID-19 in recovered patients has been increasingly reported. However, the immune mechanisms behind the recurrence have not been thoroughly investigated. The presence of neutralizing antibodies (nAbs) in recurrence/reinfection cases suggests that other types of immune response are involved in protection against recurrence. Here, we investigated the innate type I/III interferon (IFN) response, binding and nAb assays and T-cell responses to severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) with IFN gamma (IFNγ) enzyme-linked spot assay (ELISPOT) in three pairs of young adult monozygotic (MZ) twins with previous confirmed COVID-19, one of them presenting a severe recurrence four months after the initial infection. Twin studies have been of paramount importance to comprehend the immunogenetics of infectious diseases. Each MZ twin pair was previously exposed to SARS-CoV-2, as seen by clinical reports. The six individuals presented similar overall recovered immune responses except for the recurrence case, who presented a drastically reduced number of recognized SARS-CoV-2 T-cell epitopes on ELISPOT as compared to her twin sister and the other twin pairs. Our results suggest that the lack of a broad T-cell response to initial infection may have led to recurrence, emphasizing that an effective SARS-CoV-2-specific T-cell immune response is key for complete viral control and avoidance of clinical recurrence of COVID-19.
Keywords: COVID-19; T cell; immunity; recurrence; severe acute respiratory distress syndrome coronavirus 2; twins.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

