Pivotal Study Evaluating the Safety and Effectiveness of the Abre Venous Self-Expanding Stent System in Patients With Symptomatic Iliofemoral Venous Outflow Obstruction
- PMID: 35105153
- PMCID: PMC8843393
- DOI: 10.1161/CIRCINTERVENTIONS.121.010960
Pivotal Study Evaluating the Safety and Effectiveness of the Abre Venous Self-Expanding Stent System in Patients With Symptomatic Iliofemoral Venous Outflow Obstruction
Abstract
Background: Iliofemoral venous obstruction is recognized with increasing frequency as the underlying cause of lower extremity symptoms including edema, pain, skin changes, and, in advanced cases, ulceration. This study sought to evaluate the safety and effectiveness of the Abre venous self-expanding stent system for the treatment of symptomatic iliofemoral venous outflow obstruction.
Methods: The ABRE Study (A Multi-Center, Non-Randomized Study to Evaluate the Safety and Effectiveness of the Abre Venous Self-Expanding Stent System in Patients With Symptomatic Iliofemoral Venous Outflow Obstruction) is a single-arm, multicenter, prospective study that included 200 subjects from 24 global sites. The primary end points were 12-month primary patency and major adverse events within 30 days. Secondary end points included lesion and procedure success, primary-assisted and secondary patency, major adverse events, stent migration, stent fracture, and quality of life changes. End point-related adverse events and imaging studies were adjudicated by independent clinical events committee and core laboratories, respectively.
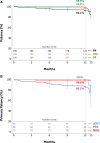
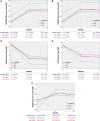
Results: Venous obstruction cause was classified as acute deep vein thrombosis (16.5%, 33/200), post-thrombotic syndrome (47.5%, 95/200), or nonthrombotic iliac vein lesion (36.0%, 72/200). The common iliac and external iliac veins were stented in 96.0% (192/200), 80.5% (161/200) of subjects, respectively. Stent implant into the common femoral vein was required in 44.0% (88/200). Primary patency at 12 months was 88.0% (162/184). Four (2.0%) major adverse events occurred within 30 days. Twelve-month primary-assisted and secondary patency were 91.8% (169/184) and 92.9% (171/184), respectively. No stent fractures or migrations were reported. Mean target limb Villalta score decreased from 11.2±5.6 at baseline to 4.1±4.8 at 12 months, and the mean target limb revised Venous Clinical Severity Score decreased from 8.8±4.7 at baseline to 4.3±3.6 at 12 months. Clinically meaningful improvements in quality of life and venous functional assessment scores from baseline were demonstrated through 12 months in all measures.
Conclusions: Symptomatic iliofemoral venous obstruction can be successfully treated with an Abre venous stent. Study outcomes demonstrated a high patency rate with a good safety profile. Patients demonstrated a significant reduction in clinical symptoms and improvement in quality of life that was maintained through 12-month follow-up. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03038438.
Keywords: iliac vein; quality of life; stent; thromboembolism; thrombosis.
Figures


References
-
- Mahnken AH, Thomson K, de Haan M, O’Sullivan GJ. CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol. 2014;37:889–897. doi: 10.1007/s00270-014-0875-4 - PubMed
-
- Esposito A, Charisis N, Kantarovsky A, Uhl JF, Labropoulos N. A comprehensive review of the pathophysiology and clinical importance of iliac vein obstruction. Eur J Vasc Endovasc Surg. 2020;60:118–125. doi: 10.1016/j.ejvs.2020.03.020 - PubMed
-
- Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990. doi: 10.1016/j.jvs.2007.06.046 - PubMed
-
- Raju S, Darcey R, Neglén P. Unexpected major role for venous stenting in deep reflux disease. J Vasc Surg. 2010;51:401–8. discussion 408. doi: 10.1016/j.jvs.2009.08.032 - PubMed
-
- Rossi FH, Kambara AM, Izukawa NM, Rodrigues TO, Rossi CB, Sousa AG, Metzger PB, Thorpe PE. Randomized double-blinded study comparing medical treatment versus iliac vein stenting in chronic venous disease. J Vasc Surg Venous Lymphat Disord. 2018;6:183–191. doi: 10.1016/j.jvsv.2017.11.003 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

