Practical "1-2-3-4-Day" Rule for Starting Direct Oral Anticoagulants After Ischemic Stroke With Atrial Fibrillation: Combined Hospital-Based Cohort Study
- PMID: 35105180
- PMCID: PMC9022681
- DOI: 10.1161/STROKEAHA.121.036695
Practical "1-2-3-4-Day" Rule for Starting Direct Oral Anticoagulants After Ischemic Stroke With Atrial Fibrillation: Combined Hospital-Based Cohort Study
Abstract
Background: The "1-3-6-12-day rule" for starting direct oral anticoagulants (DOACs) in patients with nonvalvular atrial fibrillation after acute ischemic stroke or transient ischemic attack recommends timings that may be later than used in clinical practice. We investigated more practical optimal timing of DOAC initiation according to stroke severity.
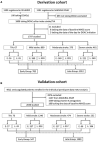
Methods: The combined data of prospective registries in Japan, Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-nonvalvular atrial fibrillation (September 2011 to March 2014) and RELAXED (February 2014 to April 2016) were used. Patients were divided into transient ischemic attack and 3 stroke subgroups by the National Institutes of Health Stroke Scale score: mild (0-7), moderate (8-15), and severe (≥16). The early treatment group was defined as patients starting DOACs earlier than the median initiation day in each subgroup. Outcomes included a composite of recurrent stroke or systemic embolism, ischemic stroke, and severe bleeding within 90 days. Six European prospective registries were used for validation.
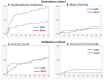
Results: In the 1797 derivation cohort patients, DOACs were started at median 2 days after transient ischemic attack and 3, 4, and 5 days after mild, moderate, and severe strokes, respectively. Stroke or systemic embolism was less common in Early Group (n=785)-initiating DOACS within 1, 2, 3, and 4 days, respectively-than Late Group (n=1012) (1.9% versus 3.9%; adjusted hazard ratio, 0.50 [95% CI, 0.27-0.89]), as was ischemic stroke (1.7% versus 3.2%, 0.54 [0.27-0.999]). Major bleeding was similarly common in the 2 groups (0.8% versus 1.0%). On validation, both ischemic stroke (2.4% versus 2.2%) and intracranial hemorrhage (0.2% versus 0.6%) were similarly common in Early (n=547) and Late (n=1483) Groups defined using derivation data.
Conclusions: In Japanese and European populations, early DOAC initiation within 1, 2, 3, or 4 days according to stroke severity seemed to be feasible to decrease the risk of recurrent stroke or systemic embolism and no increase in major bleeding. These findings support ongoing randomized trials to better establish the optimal timing of DOAC initiation.
Keywords: acute ischemic stroke; anticoagulation; atrial fibrillation; cardioembolism; stroke prevention.
Figures
References
-
- Seiffge DJ, Paciaroni M, Wilson D, Koga M, Macha K, Cappellari M, Schaedelin S, Shakeshaft C, Takagi M, Tsivgoulis G, et al. ; CROMIS-2, RAF, RAF-DOAC, SAMURAI, NOACISP LONGTERM, Erlangen and Verona registry collaborators. Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol. 2019;85:823–834. doi: 10.1002/ana.25489 - PMC - PubMed
-
- Masotti L, Grifoni E, Dei A, Vannucchi V, Moroni F, Panigada G, Spolveri S, Landini G. Direct oral anticoagulants in the early phase of non valvular atrial fibrillation-related acute ischemic stroke: focus on real life studies. J Thromb Thrombolysis. 2019;47:292–300. doi: 10.1007/s11239-018-1775-2 - PubMed
-
- Xian Y, Xu H, O’Brien EC, Shah S, Thomas L, Pencina MJ, Fonarow GC, Olson DM, Schwamm LH, Bhatt DL, et al. . Clinical effectiveness of direct oral anticoagulants vs warfarin in older patients with atrial fibrillation and ischemic stroke: findings from the Patient-Centered Research Into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) Study. JAMA Neurol. 2019;76:1192–1202. doi: 10.1001/jamaneurol.2019.2099 - PMC - PubMed
-
- Labovitz AJ, Rose DZ, Fradley MG, Meriwether JN, Renati S, Martin R, Kasprowicz T, Murtagh R, Kip K, Beckie TM, et al. ; AREST Investigators. Early apixaban use following stroke in patients with atrial fibrillation: results of the AREST Trial. Stroke. 2021;52:1164–1171. doi: 10.1161/STROKEAHA.120.030042 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

