HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management
- PMID: 35105976
- PMCID: PMC8805140
- DOI: 10.1038/s41571-022-00603-7
HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management
Abstract
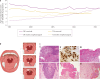
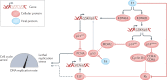
Human papillomavirus (HPV)-positive (HPV+) oropharyngeal squamous cell carcinoma (OPSCC) has one of the most rapidly increasing incidences of any cancer in high-income countries. The most recent (8th) edition of the UICC/AJCC staging system separates HPV+ OPSCC from its HPV-negative (HPV-) counterpart to account for the improved prognosis seen in the former. Indeed, owing to its improved prognosis and greater prevalence in younger individuals, numerous ongoing trials are examining the potential for treatment de-intensification as a means to improve quality of life while maintaining acceptable survival outcomes. In addition, owing to the distinct biology of HPV+ OPSCCs, targeted therapies and immunotherapies have become an area of particular interest. Importantly, OPSCC is often detected at an advanced stage owing to a lack of symptoms in the early stages; therefore, a need exists to identify and validate possible diagnostic biomarkers to aid in earlier detection. In this Review, we provide a summary of the epidemiology, molecular biology and clinical management of HPV+ OPSCC in an effort to highlight important advances in the field. Ultimately, a need exists for improved understanding of the molecular basis and clinical course of this disease to guide efforts towards early detection and precision care, and to improve patient outcomes.
© 2022. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
HPV-associated oropharyngeal cancer - discussion points.Nat Rev Clin Oncol. 2022 Jun;19(6):422. doi: 10.1038/s41571-022-00626-0. Nat Rev Clin Oncol. 2022. PMID: 35351992 No abstract available.
-
Reply to 'HPV-associated oropharyngeal cancer - discussion points'.Nat Rev Clin Oncol. 2022 Jun;19(6):422-423. doi: 10.1038/s41571-022-00627-z. Nat Rev Clin Oncol. 2022. PMID: 35351994 No abstract available.
References
-
- Lei J, et al. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 2020;383:1340–1348. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

