Iodine-Catalysed Dissolution of Elemental Gold in Ethanol
- PMID: 35106899
- PMCID: PMC9305299
- DOI: 10.1002/anie.202117587
Iodine-Catalysed Dissolution of Elemental Gold in Ethanol
Abstract
Gold is a scarce element in the Earth's crust but indispensable in modern electronic devices. New, sustainable methods of gold recycling are essential to meet the growing eco-social demand of gold. Here, we describe a simple, inexpensive, and environmentally benign dissolution of gold under mild conditions. Gold dissolves quantitatively in ethanol using 2-mercaptobenzimidazole as a ligand in the presence of a catalytic amount of iodine. Mechanistically, the dissolution of gold begins when I2 oxidizes Au0 and forms a [AuI I2 ]- species, which undergoes subsequent ligand-exchange reactions and forms a stable bis-ligand AuI complex. H2 O2 oxidizes free iodide and regenerated I2 returns back to the catalytic cycle. Addition of a reductant to the reaction mixture precipitates gold quantitatively and partially regenerates the ligand. We anticipate our work will open a new pathway to more sustainable metal recycling with the utilization of just catalytic amounts of reagents and green solvents.
Keywords: Catalysis; Gold; Iodine; Recycling; Sustainable Chemistry.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


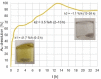
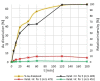
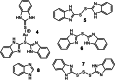
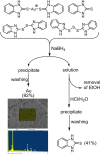
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

