Pharmacometabonomic association of cyclophosphamide 4-hydroxylation in hematopoietic cell transplant recipients
- PMID: 35106927
- PMCID: PMC9099130
- DOI: 10.1111/cts.13239
Pharmacometabonomic association of cyclophosphamide 4-hydroxylation in hematopoietic cell transplant recipients
Abstract
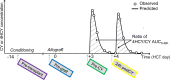
The widely used alkylating agent cyclophosphamide (CY) has substantive interpatient variability in the area under the curve (AUC) of it and its metabolites. Numerous factors may influence the drug-metabolizing enzymes that metabolize CY to 4-hydroxycyclophosphamide (4HCY), the principal precursor to CY's cytotoxic metabolite. We sought to identify endogenous metabolomics compounds (EMCs) associated with 4HCY formation clearance (ratio of 4HCY/CY AUC) using global metabolomics. Patients who undergo hematopoietic cell transplantation receiving post-transplant CY (PT-CY) were enrolled, cohort 1 (n = 26) and cohort 2 (n = 25) donating longitudinal blood samples before they started HCT (pre-HCT), before infusion of the donor allograft (pre-graft), before the first dose of PT-CY (pre-CY), and 24 h after the first dose of PT-CY (24-h post-CY), which is also immediately before the second dose of CY. A total of 512 and 498 EMCs were quantitated in two cohorts, respectively. Both univariate linear regression with false discovery rate (FDR), and pathway enrichment analyses using a global association test were performed. At the pre-CY time point, no EMCs were associated at FDR less than 0.1. At pre-HCT, cohort 1 had one EMC (levoglucosan) survive the FDR threshold. At pre-graft, cohort 1 and cohort 2 had 20 and 13 EMCs, respectively, exhibiting unadjusted p values less than 0.05, with the only EMCs having an FDR less than 0.1 being two unknown EMCs. At 24-h post-CY, there were three EMCs, two ketones, and threitol, at FDR less than 0.1 in cohort 2. These results demonstrate the potential of pharmacometabonomics, but future studies in larger samples are needed to optimize CY.
© 2022 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

