Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review
- PMID: 35106971
- PMCID: PMC8977978
- DOI: 10.1002/jcsm.12901
Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review
Abstract
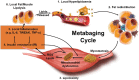
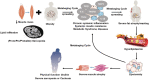
Age-associated obesity and muscle atrophy (sarcopenia) are intimately connected and are reciprocally regulated by adipose tissue and skeletal muscle dysfunction. During ageing, adipose inflammation leads to the redistribution of fat to the intra-abdominal area (visceral fat) and fatty infiltrations in skeletal muscles, resulting in decreased overall strength and functionality. Lipids and their derivatives accumulate both within and between muscle cells, inducing mitochondrial dysfunction, disturbing β-oxidation of fatty acids, and enhancing reactive oxygen species (ROS) production, leading to lipotoxicity and insulin resistance, as well as enhanced secretion of some pro-inflammatory cytokines. In turn, these muscle-secreted cytokines may exacerbate adipose tissue atrophy, support chronic low-grade inflammation, and establish a vicious cycle of local hyperlipidaemia, insulin resistance, and inflammation that spreads systemically, thus promoting the development of sarcopenic obesity (SO). We call this the metabaging cycle. Patients with SO show an increased risk of systemic insulin resistance, systemic inflammation, associated chronic diseases, and the subsequent progression to full-blown sarcopenia and even cachexia. Meanwhile in many cardiometabolic diseases, the ostensibly protective effect of obesity in extremely elderly subjects, also known as the 'obesity paradox', could possibly be explained by our theory that many elderly subjects with normal body mass index might actually harbour SO to various degrees, before it progresses to full-blown severe sarcopenia. Our review outlines current knowledge concerning the possible chain of causation between sarcopenia and obesity, proposes a solution to the obesity paradox, and the role of fat mass in ageing.
Keywords: Inflammation; Insulin resistance; Myosteatosis; Obesity; Proto-sarcopenia; Sarcopenia.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
Chun‐wei Li, Kang Yu, Ng Shyh‐Chang, Zongmin Jiang, Taoyan Liu, Shilin Ma, Lanfang Luo, Lu Guang, Kun Liang, Wenwu Ma, Hefan Miao, Wenhua Cao, Ruirui Liu, Ling‐juan Jiang, Song‐lin Yu, Chao Li, Hui‐jun Liu, Long‐yu Xu, Rong‐ji Liu, Xin‐yuan Zhang, and Gao‐shan Liu declare that they have no conflicts of interest.
Figures
References
-
- Zamboni M, Mazzali G, Fantin F, Rossi A, Francesco VD. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis Actions. 2008;18:388–395. - PubMed
-
- Kemmler W, Stengel SV, Schoene D. Longitudinal changes in muscle mass and function in older men at increased risk for sarcopenia—the FrOST‐Study. J Frailty Aging. 2019;8:57–61. - PubMed
-
- Murton AJ, Marimuthu K, Mallinson JE, Selby AL, Smith K, Rennie MJ, et al. Obesity appears to be associated with altered muscle protein synthetic and breakdown responses to increased nutrient delivery in older men, but not reduced muscle mass or contractile function. Diabetes. 2015;64:3160–3171. - PubMed
Publication types
MeSH terms
Grants and funding
- 1191957202/National Natural Science Foundation of China
- 81900782/National Natural Science Foundation of China
- XDA16010109/Strategic Priority Research Program of the CAS
- 2018YFE0201103/National Key R&D Program of China
- 2018YFC1004102/National Key R&D Program of China
- 2019YFA0801701/National Key R&D Program of China
- 2021YFE0111800/National Key R&D Program of China
- CNSNNSRG2021-129/Whole People Nutrition Research Fund
- KJZD-SW-L04/Key Research of Program of the CAS
- YSBR-012/CAS Project for Young Scientists in Basic Research
- FIRMA180301/Strategic Collaborative Research Program of the Ferring Institute of Reproductive Medicine
- FIRMA200507/Strategic Collaborative Research Program of the Ferring Institute of Reproductive Medicine
- TY0171102/Nutrition Scientific Research Foundation of BY-HEALTH
- 2017YJ023/Foundation of Tianjin Union Medical Center
LinkOut - more resources
Full Text Sources
Miscellaneous

