Insights into cisplatin-induced neurotoxicity and mitochondrial dysfunction in Caenorhabditis elegans
- PMID: 35107130
- PMCID: PMC8995082
- DOI: 10.1242/dmm.049161
Insights into cisplatin-induced neurotoxicity and mitochondrial dysfunction in Caenorhabditis elegans
Abstract
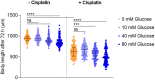
Cisplatin is the most common drug in first-line chemotherapy against solid tumors. We and others have previously used the nematode Caenorhabditis elegans to identify genetic factors influencing the sensitivity and resistance to cisplatin. In this study, we used C. elegans to explore cisplatin effects on mitochondrial functions and investigate cisplatin-induced neurotoxicity through a high-resolution system for evaluating locomotion. First, we report that a high-glucose diet sensitizes C. elegans to cisplatin at the physiological level and that mitochondrial CED-13 protects the cell from cisplatin-induced oxidative stress. Additionally, by assessing mitochondrial function with a Seahorse XFe96 Analyzer, we observed a detrimental effect of cisplatin and glucose on mitochondrial respiration. Second, because catechol-O-methyltransferases (involved in dopamine degradation) are upregulated upon cisplatin exposure, we studied the protective role of dopamine against cisplatin-induced neurotoxicity. Using a Tierpsy Tracker system for measuring neurotoxicity, we showed that abnormal displacements and body postures in cat-2 mutants, which have dopamine synthesis disrupted, can be rescued by adding dopamine. Then, we demonstrated that dopamine treatment protects against the dose-dependent neurotoxicity caused by cisplatin.
Keywords: C. elegans; CRISPR-Cas9; Cisplatin; Glucose; Mitochondria; Neurotoxicity.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests E.N. received research support from Roche, Merck Serono, Bristol Myers Squibb and Pfizer, and participated in advisory boards or lectures from Bristol Myers Squibb, Merck Serono, Merck Sharpe & Dohme, Lilly, Roche, Pfizer, Takeda, Bayer, Boehringer Ingelheim, Amgen and AstraZeneca.
Figures

References
-
- Alcántar-Fernández, J., González-Maciel, A., Reynoso-Robles, R., Pérez Andrade, M. E., Hernández-Vázquez, A. J., Velázquez-Arellano, A. and Miranda-Ríos, J. (2019). High-glucose diets induce mitochondrial dysfunction in Caenorhabditis elegans. PLoS ONE 14, e0226652. 10.1371/journal.pone.0226652 - DOI - PMC - PubMed
-
- Barone, B. B., Yeh, H. C., Snyder, C. F., Peairs, K. S., Stein, K. B., Derr, R. L., Wolff, A. C. and Brancati, F. L. (2008). Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 300, 2754-2764. 10.1001/jama.2008.824 - DOI - PMC - PubMed
-
- Bergamino, M., Rullan, A. J., Saigí, M., Peiró, I., Montanya, E., Palmero, R., Ruffinelli, J. C., Navarro, A., Arnaiz, M. D., Brao, I.et al. (2019). Fasting plasma glucose is an independent predictor of survival in patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. BMC Cancer 19, 165. 10.1186/s12885-019-5370-5 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

