SARS-CoV-2 Infection and Lung Regeneration
- PMID: 35107300
- PMCID: PMC8809385
- DOI: 10.1128/cmr.00188-21
SARS-CoV-2 Infection and Lung Regeneration
Abstract
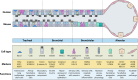
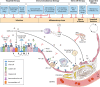
The lung is the primary site of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced immunopathology whereby the virus enters the host cells by binding to angiotensin-converting enzyme 2 (ACE2). Sophisticated regeneration and repair programs exist in the lungs to replenish injured cell populations. However, known resident stem/progenitor cells have been demonstrated to express ACE2, raising a substantial concern regarding the long-term consequences of impaired lung regeneration after SARS-CoV-2 infection. Moreover, clinical treatments may also affect lung repair from antiviral drug candidates to mechanical ventilation. In this review, we highlight how SARS-CoV-2 disrupts a program that governs lung homeostasis. We also summarize the current efforts of targeted therapy and supportive treatments for COVID-19 patients. In addition, we discuss the pros and cons of cell therapy with mesenchymal stem cells or resident lung epithelial stem/progenitor cells in preventing post-acute sequelae of COVID-19. We propose that, in addition to symptomatic treatments being developed and applied in the clinic, targeting lung regeneration is also essential to restore lung homeostasis in COVID-19 patients.
Keywords: cell therapy; lung injury; mesenchymal stem cells; post-acute sequelae of COVID-19; resident epithelial stem/progenitor cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Sungnak W, Huang N, Becavin C, Berg M, Queen R, Litvinukova M, Talavera-Lopez C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL, HCA Lung Biological Network . 2020. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26:681–687. 10.1038/s41591-020-0868-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

