FAdV-4 without Fiber-2 Is a Highly Attenuated and Protective Vaccine Candidate
- PMID: 35107364
- PMCID: PMC8809343
- DOI: 10.1128/spectrum.01436-21
FAdV-4 without Fiber-2 Is a Highly Attenuated and Protective Vaccine Candidate
Abstract
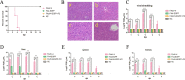
Hepatitis-hydropericardium syndrome (HHS) caused by the highly pathogenic fowl adenovirus serotype 4 (FAdV-4) has resulted in huge economic losses to the poultry industry globally. The fiber-2 gene, as a major virulence determiner, is also an important vaccine target against FAdV-4. In this study, we used a CRISPR/Cas9-based homology-dependent recombinant technique to replace the fiber-2 gene with egfp and generate a novel recombinant virus, designated FAdV4-EGFP-rF2. Although FAdV4-EGFP-rF2 showed low replication ability compared to the wild-type FAdV-4 in LMH cells, FAdV4-EGFP-rF2 could effectively replicate in LMH-F2 cells with the expression of Fiber-2. Moreover, FAdV4-EGFP-rF2 was not only highly attenuated in chickens, but also could provide efficient protection against a lethal challenge of FAdV-4. Moreover, FAdV4-EGFP-rF2 without fiber-2 could induce neutralizing antibodies at the same level as FA4-EGFP with fiber-2. These results clearly demonstrate that although fiber-2 affects the viral replication and pathogenesis of FAdV-4, it is not necessary for virus replication and induction of neutralizing antibodies; these findings provide novel insights into the roles of fiber-2 and highlight fiber-2 as an insertion site for generating live-attenuated FAdV-4 vaccines against FAdV-4 and other pathogens. IMPORTANCE Among all serotypes of fowl adenovirus, serotypes FAdV-1, FAdV-4, and FAdV-10 are unique members with two fiber genes (fiber-1 and fiber-2). Recent studies reveal that Fiber-1, not Fiber-2, directly triggers viral infection of FAdV-4, whereas Fiber-2, but not Fiber-1, has been identified as the major virulence determiner and an efficient protective immunogen for subunit vaccines. Here, we replaced fiber-2 with egfp to generate a novel recombinant virus, designated FAdV4-EGFP-rF2. In vitro and in vivo studies on FAdV4-EGFP-rF2 revealed that fiber-2 was not necessary for either virus replication or efficient protection for FAdV-4; these results not only provide a novel live-attenuated vaccine candidate against HHS, but also give new ideas for generating a FAdV-4 based vaccine vector against other pathogens.
Keywords: CRISPR/Cas9; FAdV-4; deletion of fiber-2; pathogenesis; protection; virus rescuing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hess M. 2020. Aviadenovirus infections, p 322–331. In Swayne DE, Boulianne M, Logue CM, McDougald LR, Nair V, Suarez DL (ed), Diseases of poultry, 14th ed. Wiley-Blackwell, Hoboken, NJ.
-
- Pan Q, Wang J, Gao YL, Wang Q, Cui HY, Liu CJ, Qi XL, Zhang YP, Wang YQ, Li K, Gao L, Liu AJ, Wang XM. 2020. Identification of chicken CAR homology as a cellular receptor for the emerging highly pathogenic fowl adenovirus 4 via unique binding mechanism. Emerg Microbes Infect 9:586–596. doi: 10.1080/22221751.2020.1736954. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

