Deeply conserved synteny and the evolution of metazoan chromosomes
- PMID: 35108053
- PMCID: PMC8809688
- DOI: 10.1126/sciadv.abi5884
Deeply conserved synteny and the evolution of metazoan chromosomes
Abstract
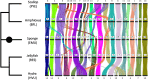
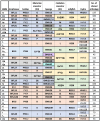
Animal genomes show networks of deeply conserved gene linkages whose phylogenetic scope and chromosomal context remain unclear. Here, we report chromosome-scale conservation of synteny among bilaterians, cnidarians, and sponges and use comparative analysis to reconstruct ancestral chromosomes across major animal groups. Comparisons among diverse metazoans reveal the processes of chromosome evolution that produced contemporary karyotypes from their Precambrian progenitors. On the basis of these findings, we introduce a simple algebraic representation of chromosomal change and use it to establish a unified systematic framework for metazoan chromosome evolution. We find that fusion-with-mixing, a previously unappreciated mode of chromosome change, has played a central role. We find that relicts of several metazoan chromosomal units are preserved in unicellular eukaryotes. These conserved pre-metazoan linkages include the chromosomal unit that encodes the most diverse set of metazoan homeobox genes, suggesting a candidate genomic context for the early diversification of this key gene family.
Figures




References
-
- Waddington C. H., Callan H. G., Animal cytology and evolution. Nature 175, 436 (1955).
-
- Putnam N. H., Srivastava M., Hellsten U., Dirks B., Chapman J., Salamov A., Terry A., Shapiro H., Lindquist E., Kapitonov V. V., Jurka J., Genikhovich G., Grigoriev I. V., Lucas S. M., Steele R. E., Finnerty J. R., Technau U., Martindale M. Q., Rokhsar D. S., Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317, 86–94 (2007). - PubMed
-
- Simakov O., Marletaz F., Cho S.-J., Edsinger-Gonzales E., Havlak P., Hellsten U., Kuo D.-H., Larsson T., Lv J., Arendt D., Savage R., Osoegawa K., de Jong P., Grimwood J., Chapman J. A., Shapiro H., Aerts A., Otillar R. P., Terry A. Y., Boore J. L., Grigoriev I. V., Lindberg D. R., Seaver E. C., Weisblat D. A., Putnam N. H., Rokhsar D. S., Insights into bilaterian evolution from three spiralian genomes. Nature 493, 526–531 (2013). - PMC - PubMed
-
- Simakov O., Kawashima T., Marlétaz F., Jenkins J., Koyanagi R., Mitros T., Hisata K., Bredeson J., Shoguchi E., Gyoja F., Yue J.-X., Chen Y.-C., Freeman R. M. Jr., Sasaki A., Hikosaka-Katayama T., Sato A., Fujie M., Baughman K. W., Levine J., Gonzalez P., Cameron C., Fritzenwanker J. H., Pani A. M., Goto H., Kanda M., Arakaki N., Yamasaki S., Qu J., Cree A., Ding Y., Dinh H. H., Dugan S., Holder M., Jhangiani S. N., Kovar C. L., Lee S. L., Lewis L. R., Morton D., Nazareth L. V., Okwuonu G., Santibanez J., Chen R., Richards S., Muzny D. M., Gillis A., Peshkin L., Wu M., Humphreys T., Su Y.-H., Putnam N. H., Schmutz J., Fujiyama A., Yu J.-K., Tagawa K., Worley K. C., Gibbs R. A., Kirschner M. W., Lowe C. J., Satoh N., Rokhsar D. S., Gerhart J., Hemichordate genomes and deuterostome origins. Nature 527, 459–465 (2015). - PMC - PubMed
-
- Putnam N. H., Butts T., Ferrier D. E. K., Furlong R. F., Hellsten U., Kawashima T., Robinson-Rechavi M., Shoguchi E., Terry A., Yu J.-K., Benito-Gutiérrez E. L., Dubchak I., Garcia-Fernàndez J., Gibson-Brown J. J., Grigoriev I. V., Horton A. C., de Jong P. J., Jurka J., Kapitonov V. V., Kohara Y., Kuroki Y., Lindquist E., Lucas S., Osoegawa K., Pennacchio L. A., Salamov A. A., Satou Y., Sauka-Spengler T., Schmutz J., Shin-I T., Toyoda A., Bronner-Fraser M., Fujiyama A., Holland L. Z., Holland P. W. H., Satoh N., Rokhsar D. S., The amphioxus genome and the evolution of the chordate karyotype. Nature 453, 1064–1071 (2008). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

