Dexamethasone sensitizes to ferroptosis by glucocorticoid receptor-induced dipeptidase-1 expression and glutathione depletion
- PMID: 35108055
- PMCID: PMC8809683
- DOI: 10.1126/sciadv.abl8920
Dexamethasone sensitizes to ferroptosis by glucocorticoid receptor-induced dipeptidase-1 expression and glutathione depletion
Abstract
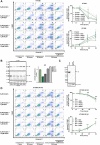
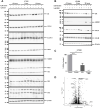
Dexamethasone is widely used as an immunosuppressive therapy and recently as COVID-19 treatment. Here, we demonstrate that dexamethasone sensitizes to ferroptosis, a form of iron-catalyzed necrosis, previously suggested to contribute to diseases such as acute kidney injury, myocardial infarction, and stroke, all of which are triggered by glutathione (GSH) depletion. GSH levels were significantly decreased by dexamethasone. Mechanistically, we identified that dexamethasone up-regulated the GSH metabolism regulating protein dipeptidase-1 (DPEP1) in a glucocorticoid receptor (GR)-dependent manner. DPEP1 knockdown reversed the phenotype of dexamethasone-induced ferroptosis sensitization. Ferroptosis inhibitors, the DPEP1 inhibitor cilastatin, or genetic DPEP1 inactivation reversed the dexamethasone-induced increase in tubular necrosis in freshly isolated renal tubules. Our data indicate that dexamethasone sensitizes to ferroptosis by a GR-mediated increase in DPEP1 expression and GSH depletion. Together, we identified a previously unknown mechanism of glucocorticoid-mediated sensitization to ferroptosis bearing clinical and therapeutic implications.
Figures



References
-
- Jackson R. K., Irving J. A., Veal G. J., Personalization of dexamethasone therapy in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 173, 13–24 (2016). - PubMed
-
- The RECOVERY Collaborative , Dexamethasone in hospitalized patients with Covid-19—Preliminary report. N. Eng. J. Med. covidwho-650223, (2020).
-
- Thomusch O., Wiesener M., Opgenoorth M., Pascher A., Woitas R. P., Witzke O., Jaenigen B., Rentsch M., Wolters H., Rath T., Cingöz T., Benck U., Banas B., Hugo C., Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): An open-label, multicentre, randomised controlled trial. Lancet 388, 3006–3016 (2016). - PubMed
-
- Moshirfar M., Somani A. N., Motlagh M. N., Ronquillo Y. C., Management of cataract in the setting of uveitis: A review of the current literature. Curr. Opin. Ophthalmol. 31, 3–9 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

