RAVAL trial: Protocol of an international, multi-centered, blinded, randomized controlled trial comparing robotic-assisted versus video-assisted lobectomy for early-stage lung cancer
- PMID: 35108265
- PMCID: PMC8809527
- DOI: 10.1371/journal.pone.0261767
RAVAL trial: Protocol of an international, multi-centered, blinded, randomized controlled trial comparing robotic-assisted versus video-assisted lobectomy for early-stage lung cancer
Abstract
Background: Retrospective data demonstrates that robotic-assisted thoracoscopic surgery provides many benefits, such as decreased postoperative pain, lower mortality, shorter length of stay, shorter chest tube duration, and reductions in the incidence of common postoperative pulmonary complications, when compared to video-assisted thoracoscopic surgery. Despite the potential benefits of robotic surgery, there are two major barriers against its widespread adoption in thoracic surgery: lack of high-quality prospective data, and the perceived higher cost of it. Therefore, in the face of these barriers, a prospective randomized controlled trial comparing robotic- to video-assisted thoracoscopic surgery is needed. The RAVAL trial is a two-phase, international, multi-centered, blinded, parallel, randomized controlled trial that is comparing robotic- to video-assisted lobectomy for early-stage non-small cell lung cancer that has been enrolling patients since 2016.
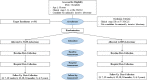
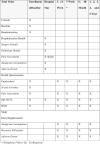
Methods: The RAVAL trial will be conducted in two phases: Phase A will enroll 186 early-stage non-small cell lung cancer patients who are candidates for minimally invasive pulmonary lobectomy; while Phase B will continue to recruit until 592 patients are enrolled. After consent, participants will be randomized in a 1:1 ratio to either robotic- or video-assisted lobectomy, and blinded to the type of surgery they are allocated to. Health-related quality of life questionnaires will be administered at baseline, postoperative day 1, weeks 3, 7, 12, months 6, 12, 18, 24, and years 3, 4, 5. The primary objective of the RAVAL trial is to determine the difference in patient-reported health-related quality of life outcomes between the robotic- and video-assisted lobectomy groups at 12 weeks. Secondary objectives include determining the differences in cost-effectiveness, and in the 5-year survival data between the two arms. The results of the primary objective will be reported once Phase A has completed accrual and the 12-month follow-ups are completed. The results of the secondary objectives will be reported once Phase B has completed accrual and the 5-year follow-ups are completed.
Discussion: If successfully completed, the RAVAL Trial will have studied patient-reported outcomes, cost-effectiveness, and survival of robotic- versus video-assisted lobectomy in a prospective, randomized, blinded fashion in an international setting.
Trial registration: ClinicalTrials.gov, NCT02617186. Registered 22-September-2015. https://clinicaltrials.gov/ct2/show/NCT02617186.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical