Fetal vs adult megakaryopoiesis
- PMID: 35108353
- PMCID: PMC9164738
- DOI: 10.1182/blood.2020009301
Fetal vs adult megakaryopoiesis
Abstract
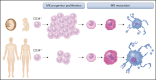
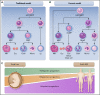
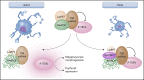
Fetal and neonatal megakaryocyte progenitors are hyperproliferative compared with adult progenitors and generate a large number of small, low-ploidy megakaryocytes. Historically, these developmental differences have been interpreted as "immaturity." However, more recent studies have demonstrated that the small, low-ploidy fetal and neonatal megakaryocytes have all the characteristics of adult polyploid megakaryocytes, including the presence of granules, a well-developed demarcation membrane system, and proplatelet formation. Thus, rather than immaturity, the features of fetal and neonatal megakaryopoiesis reflect a developmentally unique uncoupling of proliferation, polyploidization, and cytoplasmic maturation, which allows fetuses and neonates to populate their rapidly expanding bone marrow and blood volume. At the molecular level, the features of fetal and neonatal megakaryopoiesis are the result of a complex interplay of developmentally regulated pathways and environmental signals from the different hematopoietic niches. Over the past few years, studies have challenged traditional paradigms about the origin of the megakaryocyte lineage in both fetal and adult life, and the application of single-cell RNA sequencing has led to a better characterization of embryonic, fetal, and adult megakaryocytes. In particular, a growing body of data suggests that at all stages of development, the various functions of megakaryocytes are not fulfilled by the megakaryocyte population as a whole, but rather by distinct megakaryocyte subpopulations with dedicated roles. Finally, recent studies have provided novel insights into the mechanisms underlying developmental disorders of megakaryopoiesis, which either uniquely affect fetuses and neonates or have different clinical presentations in neonatal compared with adult life.
© 2022 by The American Society of Hematology.
Figures


References
-
- Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126(22):5073-5084. - PubMed
-
- Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897-906. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

