Diabetes-Induced Cellular Senescence and Senescence-Associated Secretory Phenotype Impair Cardiac Regeneration and Function Independently of Age
- PMID: 35108360
- PMCID: PMC9490451
- DOI: 10.2337/db21-0536
Diabetes-Induced Cellular Senescence and Senescence-Associated Secretory Phenotype Impair Cardiac Regeneration and Function Independently of Age
Abstract
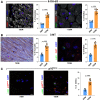
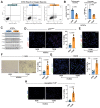
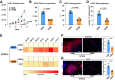
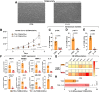
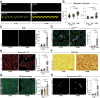
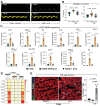
Diabetes mellitus (DM) affects the biology of multipotent cardiac stem/progenitor cells (CSCs) and adult myocardial regeneration. We assessed the hypothesis that senescence and senescence-associated secretory phenotype (SASP) are main mechanisms of cardiac degenerative defect in DM. Accordingly, we tested whether ablation of senescent CSCs would rescue the cardiac regenerative/reparative defect imposed by DM. We obtained cardiac tissue from nonaged (50- to 64-year-old) patients with type 2 diabetes mellitus (T2DM) and without DM (NDM) and postinfarct cardiomyopathy undergoing cardiac surgery. A higher reactive oxygen species production in T2DM was associated with an increased number of senescent/dysfunctional T2DM-human CSCs (hCSCs) with reduced proliferation, clonogenesis/spherogenesis, and myogenic differentiation versus NDM-hCSCs in vitro. T2DM-hCSCs showed a defined pathologic SASP. A combination of two senolytics, dasatinib (D) and quercetin (Q), cleared senescent T2DM-hCSCs in vitro, restoring their expansion and myogenic differentiation capacities. In a T2DM model in young mice, diabetic status per se (independently of ischemia and age) caused CSC senescence coupled with myocardial pathologic remodeling and cardiac dysfunction. D + Q treatment efficiently eliminated senescent cells, rescuing CSC function, which resulted in functional myocardial repair/regeneration, improving cardiac function in murine DM. In conclusion, DM hampers CSC biology, inhibiting CSCs' regenerative potential through the induction of cellular senescence and SASP independently from aging. Senolytics clear senescence, abrogating the SASP and restoring a fully proliferative/differentiation-competent hCSC pool in T2DM with normalization of cardiac function.
© 2022 by the American Diabetes Association.
Figures


References
-
- Dunlay SM, Givertz MM, Aguilar D, et al. .; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing and the Heart Failure Society of America . Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America: This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation 2019;140:e294–e324 - PubMed
-
- Gude NA, Broughton KM, Firouzi F, Sussman MA. Cardiac ageing: extrinsic and intrinsic factors in cellular renewal and senescence. Nat Rev Cardiol 2018;15:523–542 - PubMed
-
- Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr 2019;13:1165–1172 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

