FGF21 suppresses alcohol consumption through an amygdalo-striatal circuit
- PMID: 35108517
- PMCID: PMC9093612
- DOI: 10.1016/j.cmet.2021.12.024
FGF21 suppresses alcohol consumption through an amygdalo-striatal circuit
Abstract
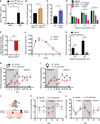
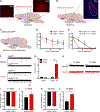
Excessive alcohol consumption is a major health and social issue in our society. Pharmacologic administration of the endocrine hormone fibroblast growth factor 21 (FGF21) suppresses alcohol consumption through actions in the brain in rodents, and genome-wide association studies have identified single nucleotide polymorphisms in genes involved with FGF21 signaling as being associated with increased alcohol consumption in humans. However, the neural circuit(s) through which FGF21 signals to suppress alcohol consumption are unknown, as are its effects on alcohol consumption in higher organisms. Here, we demonstrate that administration of an FGF21 analog to alcohol-preferring non-human primates reduces alcohol intake by 50%. Further, we reveal that FGF21 suppresses alcohol consumption through a projection-specific subpopulation of KLB-expressing neurons in the basolateral amygdala. Our results illustrate how FGF21 suppresses alcohol consumption through a specific population of neurons in the brain and demonstrate its therapeutic potential in non-human primate models of excessive alcohol consumption.
Keywords: FGF21; alcohol; basolateral amygdala; betaklotho; brain; hepatokine; liver; nucleus accumbens.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests Recombinant human FGF21 protein was provided by Novo Nordisk, and PF-05231023 was provided by Pfizer. Neither Novo Nordisk nor Pfizer was involved with the conceptualization, design, data collection, analysis, or preparation of the manuscript. Correspondence and requests for materials should be addressed to M.J.P.
Figures


Comment in
-
FGF21 regulates alcohol intake: New hopes on the rise for alcohol use disorder treatment?Cell Rep Med. 2022 Mar 15;3(3):100578. doi: 10.1016/j.xcrm.2022.100578. eCollection 2022 Mar 15. Cell Rep Med. 2022. PMID: 35492877 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

