Melatonin alleviates lipopolysaccharide (LPS) / adenosine triphosphate (ATP)-induced pyroptosis in rat alveolar Type II cells (RLE-6TN) through nuclear factor erythroid 2-related factor 2 (Nrf2)-driven reactive oxygen species (ROS) downregulation
- PMID: 35109747
- PMCID: PMC8973817
- DOI: 10.1080/21655979.2021.2018981
Melatonin alleviates lipopolysaccharide (LPS) / adenosine triphosphate (ATP)-induced pyroptosis in rat alveolar Type II cells (RLE-6TN) through nuclear factor erythroid 2-related factor 2 (Nrf2)-driven reactive oxygen species (ROS) downregulation
Abstract
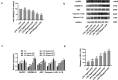
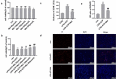
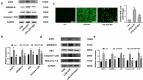
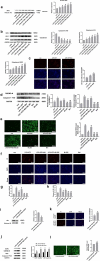
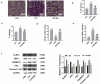
Pyroptosis has pivotal parts within disease development, rendering this attractive mechanism for novel therapeutics. This investigation aimed at analyzing melatonin roles within pyroptosis together with related mechanistics. RLE-6TN cultures were exposed to varying LPS doses for 4.5 h followed by concomitant culturing in the presence of ATP (5 mM) for 0.5 h to induce injury, and the roles of melatonin, N-Acety-L-cysteine (NAC - a ROS scavenger), ML385 (specific Nrf2 inhibitor) were examined. Apoptosis analysis was performed through lactate dehydrogenase (LDH) activity assays, together with propidium iodide (PI) stain-assay. Intracellular ROS were quantified through 2, 7-dichlorodihydrofluorescein diacetate (DCFH-DA). Pyrolysis-associated proteins, such as nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3), apoptosis-associated speck-like protein containing a CARD (ASC), cysteine aspartate-specific protease-1 P20 (Caspase-1 P20), gasdermin D-N (GSDMD-N), and mature interleukin-1β (IL-1β), were identified through Western blotting. Dataset outcomes demonstrated LPS/ATP induce RLE-6TN cell pyroptosis, while melatonin alleviated this phenomenon, visualized through increased cell survival rate, reduction of LDH discharge and PI+ cellular count. Moreover, melatonin effectively reduced NLRP3 inflammasome triggering in RLE-6TN cells. Meanwhile, this study demonstrated melatonin thwarting over NLRP3 inflammasome triggering was depending on ROS. In addition, this study found that melatonin activated Nrf2/Heme Oxygenase-1 (HO-1) pathway, with pyroptotic-inhibiting function of melatonin was reverted through a bespoke Nrf2-inhibitor and siNrf2. In summary, this study concluded that melatonin prevents RLE-6TN cellular pyroptosis through Nrf2-triggered ROS downregulation.
Keywords: Melatonin; NLRP3 inflammasome; Nrf2; RLE-6TN; ROS; pyroptosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Shao XF, Li B, Shen J, et al. Ghrelin alleviates traumatic brain injury-induced acute lung injury through pyroptosis/NF-κB pathway. Int Immunopharmacol. 2020;79:106175. - PubMed
-
- Lamkanfi M, Dixit VM.. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
