Ketamine for the acute treatment of severe suicidal ideation: double blind, randomised placebo controlled trial
- PMID: 35110300
- PMCID: PMC8808464
- DOI: 10.1136/bmj-2021-067194
Ketamine for the acute treatment of severe suicidal ideation: double blind, randomised placebo controlled trial
Abstract
Objective: To confirm the rapid onset anti-suicidal benefits of ketamine in the short term and at six weeks, overall and according to diagnostic group.
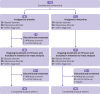
Design: Prospective, double blind, superiority, randomised placebo controlled trial.
Setting: Seven French teaching hospitals between 13 April 2015 and 12 March 2019.
Eligibility criteria for participants: Aged 18 or older with current suicidal ideation, admitted to hospital voluntarily. Exclusion criteria included a history of schizophrenia or other psychotic disorders, substance dependence, and contraindications for ketamine.
Participants: 156 participants were recruited and randomised to placebo (n=83) or ketamine (n=73), stratified by centre and diagnosis: bipolar, depressive, or other disorders.
Intervention: Two 40 minute intravenous infusions of ketamine (0.5 mg/kg) or placebo (saline) were administered at baseline and 24 hours, in addition to usual treatment.
Main outcome measures: The primary outcome was the rate of patients in full suicidal remission at day 3, according to the scale for suicidal ideation total score ≤3. Analyses were conducted on an intention-to-treat basis.
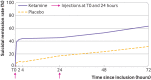
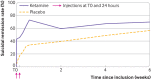
Results: More participants receiving ketamine reached full remission of suicidal ideas at day 3 than those receiving placebo: 46 (63.0%) of 83 participants in the ketamine arm and 25 (31.6%) of 73 in the placebo arm (odds ratio 3.7 (95% confidence interval 1.9 to 7.3), P<0.001). This effect differed according to the diagnosis (treatment: P<0.001; interaction: P=0.02): bipolar (odds ratio 14.1 (95% confidence interval 3.0 to 92.2), P<0.001), depressive (1.3 (0.3 to 5.2), P=0.6), or other disorders (3.7 (0.9 to 17.3, P=0.07)). Side effects were limited. No manic or psychotic symptom was seen. Moreover, a mediating effect of mental pain was found. At week 6, remission in the ketamine arm remained high, although non-significantly versus placebo (69.5% v 56.3%; odds ratio 0.8 (95% confidence interval 0.3 to 2.5), P=0.7).
Conclusions: The findings indicate that ketamine is rapid, safe in the short term, and has persistent benefits for acute care in suicidal patients. Comorbid mental disorders appear to be important moderators. An analgesic effect on mental pain might explain the anti-suicidal effects of ketamine.
Trial registration: ClinicalTrials.gov NCT02299440.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from PHRC-N 2013 for the submitted work; FJ has no declaration of interests for the past five years. PG received, during the past five years, fees for presentations at congresses or participation in scientific boards from Alcediag-Alcen, Angelini, GSK, Janssen, Lundbeck, Otsuka, SAGE, and Servier. WE-H reports personal fees from EISAI, Janssen, Lundbeck, Otsuka, UCB, and Chugai. PC received speaker and consultation fees from Exeltis, Janssen, and Pfizer. GV is part of a scientific board for Janssen. RG has received compensation as a member of the scientific advisory board of Janssen, Lundbeck, Roche, SOBI, and Takeda; he has served as consultant and/or speaker for Astra Zeneca, Boehringer-Ingelheim, Pierre Fabre, Lilly, Lundbeck, MAPREG, Otsuka, Pileje, SANOFI, Servier, LVMH and received compensation; and he has received research support from Servier; cofounder and stock shareholder: Regstem. P-ML has received compensation as consultant and member of a scientific advisory board for Janssen. LS declares fees for advisory board, travel support activities of consultant and lecturer in the past five years received from Janssen, Lundbeck, and Otsuka; fees for advisory board, travel support activities of consultant, lecturer, and faculty member in the past five years received from Eisai, Janssen, Lundbeck, Otsuka, Sanofi, Teva. MA declares fees from Astra Zeneca and Lundbeck, and has been invited to congresses by Janssen-Cilag, Otsuka, Lundbeck, Servier, and Astra Zeneca.
Figures

Comment in
-
Ketamine for suicidal ideation: heed lessons from opiate epidemic.BMJ. 2022 Mar 8;376:o597. doi: 10.1136/bmj.o597. BMJ. 2022. PMID: 35260394 No abstract available.
References
-
- World Health Statistics. Monitoring health for sustainable development goals. 2016.
-
- Kennedy SH, Lam RW, McIntyre RS, et al. CANMAT Depression Work Group . Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Can J Psychiatry 2016;61:540-60. 10.1177/0706743716659417 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
