Randomised controlled trial of Advanced Hybrid Closed Loop in an Adult Population with Type 1 Diabetes (ADAPT): study protocol and rationale
- PMID: 35110310
- PMCID: PMC8811581
- DOI: 10.1136/bmjopen-2021-050635
Randomised controlled trial of Advanced Hybrid Closed Loop in an Adult Population with Type 1 Diabetes (ADAPT): study protocol and rationale
Abstract
Introduction: For many people with type 1 diabetes who struggle to achieve glycaemic control with multiple daily injections of insulin (MDI) plus self-monitoring of blood glucose, MDI plus intermittently scanned continuous glucose monitoring (IS-CGM) or real-time continuous glucose monitoring (RT-CGM), or insulin administration using insulin pump therapy represent optimised care in many regions. Through technological advances an advanced hybrid closed loop (AHCL) system has been developed; studies of incremental effects relative to MDI plus IS-CGM are lacking.
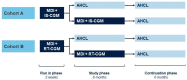
Methods and analysis: The Advanced Hybrid Closed Loop study in Adult Population with Type 1 Diabetes (ADAPT) study is a multinational, prospective, open-label, confirmatory and exploratory randomised controlled trial to examine outcomes with the MiniMed 670G version 4.0 AHCL system (with an equivalent algorithm and commercialised as the MiniMed 780G system, referred to as AHCL) relative to MDI plus IS-CGM in adults with baseline HbA1c≥8.0%. An exploratory cohort will compare AHCL with MDI plus RT-CGM. The study will be conducted in approximately 124 adults on MDI plus either IS-CGM or RT-CGM for at least 3 months prior to screening. The primary endpoint will be the difference in mean HbA1c change from baseline to 6 months between the AHCL and the MDI plus IS-CGM arms. Secondary endpoints will include proportion of time spent in hypoglycaemic, euglycaemic and hyperglycaemic ranges.
Ethics and dissemination: The ADAPT study will be conducted in accordance with the requirements of the Declaration of Helsinki and local laws and regulations, and has been approved by ethics committees. The trial will provide valuable information on the incremental benefits that may be provided by AHCL for patients failing to achieve glycaemic targets on MDI plus IS-CGM or RT-CGM and form a basis for health economic evaluations to support market access.
Trial registration number: NCT04235504; Pre-results.
Keywords: diabetes & endocrinology; general diabetes.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SdP, LV, RR, JS, AH, JC and OC are employees of Medtronic.
Figures
Similar articles
-
Advanced Hybrid Closed Loop in Adult Population With Type 1 Diabetes: A Substudy From the ADAPT Randomized Controlled Trial in Users of Real-Time Continuous Glucose Monitoring.J Diabetes Sci Technol. 2024 Sep;18(5):1132-1138. doi: 10.1177/19322968231161320. Epub 2023 Mar 22. J Diabetes Sci Technol. 2024. PMID: 36949671 Free PMC article. Clinical Trial.
-
A European Cost-Utility Analysis of the MiniMed™ 780G Advanced Hybrid Closed-Loop System Versus Intermittently Scanned Continuous Glucose Monitoring with Multiple Daily Insulin Injections in People Living with Type 1 Diabetes.Diabetes Technol Ther. 2023 Dec;25(12):864-876. doi: 10.1089/dia.2023.0297. Diabetes Technol Ther. 2023. PMID: 37801658
-
Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study.Lancet Diabetes Endocrinol. 2022 Oct;10(10):720-731. doi: 10.1016/S2213-8587(22)00212-1. Epub 2022 Sep 1. Lancet Diabetes Endocrinol. 2022. PMID: 36058207 Clinical Trial.
-
Use of continuous glucose monitoring and hybrid closed-loop therapy in pregnancy.Diabetes Obes Metab. 2024 Dec;26 Suppl 7:74-91. doi: 10.1111/dom.15999. Epub 2024 Oct 16. Diabetes Obes Metab. 2024. PMID: 39411880 Review.
-
Optimizing type 1 diabetes after multiple daily injections and capillary blood monitoring: Pump or sensor first? A meta-analysis using pooled differences in outcome measures.Diabetes Obes Metab. 2021 Nov;23(11):2521-2528. doi: 10.1111/dom.14498. Epub 2021 Aug 12. Diabetes Obes Metab. 2021. PMID: 34286892
Cited by
-
Advanced Hybrid Closed Loop in Adult Population With Type 1 Diabetes: A Substudy From the ADAPT Randomized Controlled Trial in Users of Real-Time Continuous Glucose Monitoring.J Diabetes Sci Technol. 2024 Sep;18(5):1132-1138. doi: 10.1177/19322968231161320. Epub 2023 Mar 22. J Diabetes Sci Technol. 2024. PMID: 36949671 Free PMC article. Clinical Trial.
-
Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control.Diabetes Metab J. 2023 Jan;47(1):27-41. doi: 10.4093/dmj.2022.0271. Epub 2023 Jan 12. Diabetes Metab J. 2023. PMID: 36635028 Free PMC article. Review.
References
-
- Collyns OJ, Meier RA, Betts ZL, et al. . Improved glycemic outcomes with medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose Suspend in people with type 1 diabetes. Diabetes Care 2021;44:dc202250. 10.2337/dc20-2250 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical