Mice expressing fluorescent PAR2 reveal that endocytosis mediates colonic inflammation and pain
- PMID: 35110404
- PMCID: PMC8833192
- DOI: 10.1073/pnas.2112059119
Mice expressing fluorescent PAR2 reveal that endocytosis mediates colonic inflammation and pain
Erratum in
-
Correction for Latorre et al., Mice expressing fluorescent PAR2 reveal that endocytosis mediates colonic inflammation and pain.Proc Natl Acad Sci U S A. 2022 Jul 19;119(29):e2209800119. doi: 10.1073/pnas.2209800119. Epub 2022 Jul 5. Proc Natl Acad Sci U S A. 2022. PMID: 35787187 Free PMC article. No abstract available.
Abstract
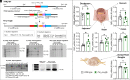
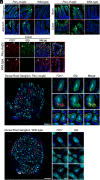
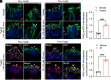
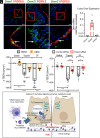
G protein-coupled receptors (GPCRs) regulate many pathophysiological processes and are major therapeutic targets. The impact of disease on the subcellular distribution and function of GPCRs is poorly understood. We investigated trafficking and signaling of protease-activated receptor 2 (PAR2) in colitis. To localize PAR2 and assess redistribution during disease, we generated knockin mice expressing PAR2 fused to monomeric ultrastable green fluorescent protein (muGFP). PAR2-muGFP signaled and trafficked normally. PAR2 messenger RNA was detected at similar levels in Par2-mugfp and wild-type mice. Immunostaining with a GFP antibody and RNAScope in situ hybridization using F2rl1 (PAR2) and Gfp probes revealed that PAR2-muGFP was expressed in epithelial cells of the small and large intestine and in subsets of enteric and dorsal root ganglia neurons. In healthy mice, PAR2-muGFP was prominently localized to the basolateral membrane of colonocytes. In mice with colitis, PAR2-muGFP was depleted from the plasma membrane of colonocytes and redistributed to early endosomes, consistent with generation of proinflammatory proteases that activate PAR2 PAR2 agonists stimulated endocytosis of PAR2 and recruitment of Gαq, Gαi, and β-arrestin to early endosomes of T84 colon carcinoma cells. PAR2 agonists increased paracellular permeability of colonic epithelial cells, induced colonic inflammation and hyperalgesia in mice, and stimulated proinflammatory cytokine release from segments of human colon. Knockdown of dynamin-2 (Dnm2), the major colonocyte isoform, and Dnm inhibition attenuated PAR2 endocytosis, signaling complex assembly and colonic inflammation and hyperalgesia. Thus, PAR2 endocytosis sustains protease-evoked inflammation and nociception and PAR2 in endosomes is a potential therapeutic target for colitis.
Keywords: endocytosis; inflammation; proteases; receptors; signaling.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: N.W.B. is a founding scientist of Endosome Therapeutics, Inc. Research in the laboratories of N.W.B., N.A.V., and D.P.P. is funded in part by Takeda Pharmaceuticals International.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

