Variation of TNF modulates cellular immunity of gregarious and solitary locusts against fungal pathogen Metarhizium anisopliae
- PMID: 35110413
- PMCID: PMC8833202
- DOI: 10.1073/pnas.2120835119
Variation of TNF modulates cellular immunity of gregarious and solitary locusts against fungal pathogen Metarhizium anisopliae
Abstract
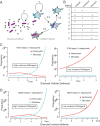
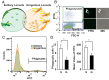
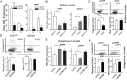
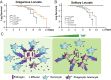
Changes in population density lead to phenotypic differentiation of solitary and gregarious locusts, which display different resistance to fungal pathogens; however, how to regulate their cellular immune strategies remains unknown. Here, our stochastic simulation of pathogen proliferation suggested that humoral defense always enhanced resistance to fungal pathogens, while phagocytosis sometimes reduced defense against pathogens. Further experimental data proved that gregarious locusts had significantly decreased phagocytosis of hemocytes compared to solitary locusts. Additionally, transcriptional analysis showed that gregarious locusts promoted immune effector expression (gnbp1 and dfp) and reduced phagocytic gene expression (eater) and the cytokine tumor necrosis factor (TNF). Interestingly, higher expression of the cytokine TNF in solitary locusts simultaneously promoted eater expression and inhibited gnbp1 and dfp expression. Moreover, inhibition of TNF increased the survival of solitary locusts, and injection of TNF decreased the survival of gregarious locusts after fungal infection. Therefore, our results indicate that the alerted expression of TNF regulated the immune strategy of locusts to adapt to environmental changes.
Keywords: cellular defenses; density-dependent prophylaxis; ecological immunology; tumor necrosis factor.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

