Organocatalytic cycloaddition of alkynylindoles with azonaphthalenes for atroposelective construction of indole-based biaryls
- PMID: 35110529
- PMCID: PMC8810779
- DOI: 10.1038/s41467-022-28211-0
Organocatalytic cycloaddition of alkynylindoles with azonaphthalenes for atroposelective construction of indole-based biaryls
Abstract
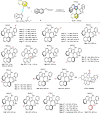
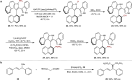
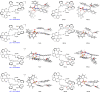
The axially chiral indole-aryl motifs are present in natural products and biologically active compounds as well as in chiral ligands. Atroposelective indole formation is an efficient method to construct indole-based biaryls. We report herein the result of a chiral phosphoric acid catalyzed asymmetric cycloaddition of 3-alkynylindoles with azonaphthalenes. A class of indole-based biaryls were prepared efficiently with excellent yields and enantioselectivities (up to 98% yield, 99% ee). Control experiment and DFT calculations illustrate a possible mechanism in which the reaction proceeds via a dearomatization of indole to generate an allene-iminium intermediate, followed by an intramolecular aza-Michael addition. This approach provides a convergent synthetic strategy for enantioselective construction of axially chiral heterobiaryl backbones.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

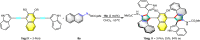

Similar articles
-
Advances in Atroposelectively De Novo Synthesis of Axially Chiral Heterobiaryl Scaffolds.Molecules. 2022 Dec 3;27(23):8517. doi: 10.3390/molecules27238517. Molecules. 2022. PMID: 36500610 Free PMC article. Review.
-
Construction of Axially Chiral Compounds via Asymmetric Organocatalysis.Acc Chem Res. 2018 Feb 20;51(2):534-547. doi: 10.1021/acs.accounts.7b00602. Epub 2018 Feb 8. Acc Chem Res. 2018. PMID: 29419282
-
Organocatalytic Atroposelective Construction of Pentatomic Heterobiaryl Diamines through Arylation of 5-Aminoisoxazoles with Azonaphthalenes.Org Lett. 2024 Apr 5;26(13):2564-2568. doi: 10.1021/acs.orglett.4c00440. Epub 2024 Mar 21. Org Lett. 2024. PMID: 38514236
-
Organocatalytic atroposelective construction of axially chiral arylquinones.Nat Commun. 2019 Sep 19;10(1):4268. doi: 10.1038/s41467-019-12269-4. Nat Commun. 2019. PMID: 31537811 Free PMC article.
-
Transition-metal-catalyzed atroposelective synthesis of axially chiral styrenes.Chem Commun (Camb). 2023 Oct 24;59(85):12669-12684. doi: 10.1039/d3cc03592a. Chem Commun (Camb). 2023. PMID: 37807950 Review.
Cited by
-
Advances in Atroposelectively De Novo Synthesis of Axially Chiral Heterobiaryl Scaffolds.Molecules. 2022 Dec 3;27(23):8517. doi: 10.3390/molecules27238517. Molecules. 2022. PMID: 36500610 Free PMC article. Review.
-
Catalytic Asymmetric Diastereodivergent Synthesis of 2-Alkenylindoles Bearing both Axial and Central Chirality.Precis Chem. 2024 Apr 23;2(5):208-220. doi: 10.1021/prechem.4c00008. eCollection 2024 May 27. Precis Chem. 2024. PMID: 39474410 Free PMC article.
-
Recent advances in organocatalytic atroposelective reactions.Beilstein J Org Chem. 2025 Jan 9;21:55-121. doi: 10.3762/bjoc.21.6. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 39811683 Free PMC article. Review.
-
Regiodivergence in the Cycloadditions between a Cyclic Nitrone and Carbonyl-Type Dipolarophiles.J Org Chem. 2025 Aug 8;90(31):11020-11032. doi: 10.1021/acs.joc.5c00727. Epub 2025 Jul 29. J Org Chem. 2025. PMID: 40730878 Free PMC article.
-
Dynamic Kinetic Resolution of Indole-Based Sulfenylated Heterobiaryls by Rhodium-Catalyzed Atroposelective Reductive Aldol Reaction.ACS Catal. 2023 Aug 30;13(18):12134-12141. doi: 10.1021/acscatal.3c03422. eCollection 2023 Sep 15. ACS Catal. 2023. PMID: 37745194 Free PMC article.
References
-
- Tang W, Zhang X. New chiral phosphorus ligands for enantioselective hydrogenation. Chem. Rev. 2003;103:3029–3070. - PubMed
-
- Bringmann G, Gulder T, Gulder TAM, Breuning M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 2011;111:563–639. - PubMed
-
- Wencel-Delord J, Panossian A, Leroux FR, Colobert F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 2015;44:3418–3430. - PubMed
-
- Wang Y-B, Tan B. Construction of axially chiral compounds via asymmetric organocatalysis. Acc. Chem. Res. 2018;51:534–547. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

