Neoantigen cancer vaccine augments anti-CTLA-4 efficacy
- PMID: 35110563
- PMCID: PMC8810847
- DOI: 10.1038/s41541-022-00433-9
Neoantigen cancer vaccine augments anti-CTLA-4 efficacy
Abstract
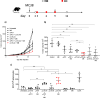
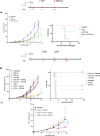
Immune checkpoint inhibitors (ICI) based on anti-CTLA-4 (αCTLA-4) and anti-PD1 (αPD1) are being tested in combination with different therapeutic approaches including other immunotherapies such as neoantigen cancer vaccines (NCV). Here we explored, in two cancer murine models, different therapeutic combinations of ICI with personalized DNA vaccines expressing neoantigens and delivered by electroporation (EP). Anti-cancer efficacy was evaluated using vaccines with or without CD4 epitopes. Therapeutic DNA vaccines showed synergistic effects in different therapeutic protocols including established large tumors. Flow cytometry (FC) was utilized to measure CD8, CD4, Treg, and switched B cells as well as neoantigen-specific immune responses, which were also measured by IFN-γ ELIspot. Immune responses were augmented in combination with αCTLA4 but not with αPD1 in the MC38 tumor-bearing mice, significantly impacting tumor growth. Similarly, neoantigen-specific T cell immune responses were enhanced in combined treatment with αCTLA-4 in the CT26 tumor model where large tumors regressed in all mice, while monotherapy with αCTLA-4 was less efficacious. In line with previous evidence, we observed an increased switched B cells in the spleen of mice treated with αCTLA-4 alone or in combination with NCV. These results support the use of NCV delivered by DNA-EP with αCTLA-4 and suggest a new combined therapy for clinical testing.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Cancer research institute. https://www.cancerresearch.org/scientists/immuno-oncology-landscape/fda-....
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

