Comparing Geant4 physics models for proton-induced dose deposition and radiolysis enhancement from a gold nanoparticle
- PMID: 35110613
- PMCID: PMC8810973
- DOI: 10.1038/s41598-022-05748-0
Comparing Geant4 physics models for proton-induced dose deposition and radiolysis enhancement from a gold nanoparticle
Abstract
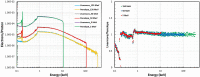
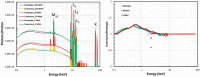
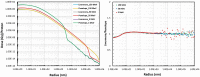
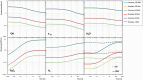
Gold nanoparticles (GNPs) are materials that make the tumor cells more radiosensitive when irradiated with ionizing radiation. The present study aimed to evaluate the impact of different physical interaction models on the dose calculations and radiochemical results around the GNP. By applying the Geant4 Monte Carlo (MC) toolkit, a single 50-nm GNP was simulated, which was immersed in a water phantom and irradiated with 5, 50, and 150 MeV proton beams. The present work assessed various parameters including the secondary electron spectra, secondary photon spectra, radial dose distribution (RDD), dose enhancement factor (DEF), and radiochemical yields around the GNP. The results with an acceptable statistical uncertainty of less than 1% indicated that low-energy electrons deriving from the ionization process formed a significant part of the total number of secondary particles generated in the presence of GNP; the Penelope model produced a larger number of these electrons by a factor of about 30%. Discrepancies of the secondary electron spectrum between Livermore and Penelope were more obvious at energies of less than 1 keV and reached the factor of about 30% at energies between 250 eV and 1 keV. The RDDs for Livermore and Penelope models were very similar with small variations within the first 6 nm from NP surface by a factor of 10%. In addition, neither the G-value nor the REF was affected by the choice of physical interaction models with the same energy cut-off. This work illustrated the similarity of the Livermore and Penelope models (within 15%) available in Geant4 for future simulation studies of GNP enhanced proton therapy with physical, physicochemical, and chemical mechanisms.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Lederman M. The early history of radiotherapy: 1895–1939. Int. J. Radiat. Oncol. Biol. Phys. 1981;7:639–648. - PubMed
-
- Kwatra D, Venugopal A, Anant S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl Cancer Res. 2013;2:330–342.
-
- Paganetti H, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002;53:407–421. - PubMed
-
- Paganetti H. Proton Therapy Physics. CRC Press; 2018.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

