Pathway-specific TNF-mediated metaplasticity in hippocampal area CA1
- PMID: 35110639
- PMCID: PMC8810872
- DOI: 10.1038/s41598-022-05844-1
Pathway-specific TNF-mediated metaplasticity in hippocampal area CA1
Abstract
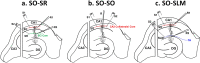
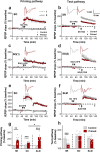
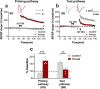
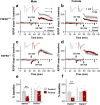
Long-term potentiation (LTP) is regulated in part by metaplasticity, the activity-dependent alterations in neural state that coordinate the direction, amplitude, and persistence of future synaptic plasticity. Previously, we documented a heterodendritic metaplasticity effect whereby high-frequency priming stimulation in stratum oriens (SO) of hippocampal CA1 suppressed subsequent LTP in the stratum radiatum (SR). The cytokine tumor necrosis factor (TNF) mediated this heterodendritic metaplasticity in wild-type rodents and in a mouse model of Alzheimer's disease. Here, we investigated whether LTP at other afferent synapses to CA1 pyramidal cells were similarly affected by priming stimulation. We found that priming stimulation in SO inhibited LTP only in SR and not in a second independent pathway in SO, nor in stratum lacunosum moleculare (SLM). Synapses in SR were also more sensitive than SO or SLM to the LTP-inhibiting effects of pharmacological TNF priming. Neither form of priming was sex-specific, while the metaplasticity effects were absent in TNFR1 knock-out mice. Our findings demonstrate an unexpected pathway specificity for the heterodendritic metaplasticity in CA1. That Schaffer collateral/commissural synapses in SR are particularly susceptible to such metaplasticity may reflect an important control of information processing in this pathway in addition to its sensitivity to neuroinflammation under disease conditions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Abraham WC, Bear MF. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. - PubMed
-
- Abraham WC. Metaplasticity: Tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 2008;9:387. - PubMed
-
- Butler MP, O'Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-alpha inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early-but not late-phase LTP. Neuroscience. 2004;124:319–326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

