"The Bitter Truth of Sugar"-Euglycemic Diabetic Ketoacidosis due to Sodium-glucose Cotransporter-2 Inhibitors: A Case Series
- PMID: 35110855
- PMCID: PMC8783253
- DOI: 10.5005/jp-journals-10071-24076
"The Bitter Truth of Sugar"-Euglycemic Diabetic Ketoacidosis due to Sodium-glucose Cotransporter-2 Inhibitors: A Case Series
Abstract
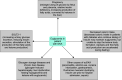
Diabetic ketoacidosis (DKA) is an acute and major complication of diabetes mellitus (DM), both type I and type II. Biochemically, DKA consists of a triad of blood sugar levels greater than 250 mg/dL, ketonemia of greater than 3 mmol/L and/or significant ketonuria, and a blood pH less than 7.3 with an increased anion gap. Currently, the sodium-glucose cotransporter-2 inhibitors (SGLT-2i) are widely used in management of type II diabetes. There have been several reports of an association between euglycemic diabetic ketoacidosis (EuDKA) and SGLT-2i agents. We present three different patients who were on SGLT-2i therapy who developed recurrent EuDKA postprocedure or sepsis. We believe that prolonged treatment (5-6 days) with intravenous (IV) insulin with glucose until resolution of glycosuria can be considered as an inexpensive marker of resolution of EuDKA. Moreover, the recommended duration for discontinuation of these drugs prior to elective procedures should be longer than 3 days. How to cite this article: Shah M, Pathrose E, Bhagwat NM, Chandy D. "The Bitter Truth of Sugar"-Euglycemic Diabetic Ketoacidosis due to Sodium-glucose Cotransporter-2 Inhibitors: A Case Series. Indian J Crit Care Med 2022;26(1):123-126.
Keywords: Euglycemia; Gliflozins; Glycosuria; High anion gap metabolic acidosis; Intensive care unit; Oral hypoglycemic agents; Sodium-glucose cotransporter-2 inhibitors.
Copyright © 2022; The Author(s).
Conflict of interest statement
Source of support: Nil Conflict of interest: None
Figures
References
-
- FDA Drug Safety Communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm. [[Last accessed on April 15, 2021]]. http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm Available from:
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous