Use of an electronic medication management support system in patients with polypharmacy in general practice: study protocol of a quantitative process evaluation of the AdAM trial
- PMID: 35111292
- PMCID: PMC8796070
- DOI: 10.1177/20420986211073215
Use of an electronic medication management support system in patients with polypharmacy in general practice: study protocol of a quantitative process evaluation of the AdAM trial
Abstract
Background: Interventional studies on polypharmacy often fail to significantly improve patient-relevant outcomes, or confine themselves to measuring surrogate parameters. Interventions and settings are complex, with many factors affecting results. The AdAM study's aim is to reduce hospitalization and death by requiring general practitioners (GPs) to use a computerized decision-support system (CDSS). The study will undergo a process evaluation to identify factors for successful implementation and to assess whether the intervention was implemented as intended.
Objective: To evaluate our complex intervention, based on the Medical Research Council's guideline dimensions.
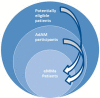
Research questions: We will assess implementation (reach, fidelity, dose, tailoring) by asking: (1) Who took part in the intervention (proportion of GPs using the CDSS, proportion of patients enrolled in them)? Information on GPs' and patients' characteristics will also be collected. (2) How many and which medication alerts were dealt with? (3) Was the intervention implemented as intended? (4) On what days did GPs use the intervention tool?
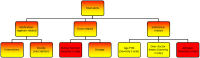
Methods: The process evaluation is part of a stepped-wedge cluster-randomized controlled trial. Characteristics of practices, GPs and patients using the CDSS will be compared with the non-participating population. CDSS log data will be analyzed to evaluate how the number of medication alerts changed between baseline and 2 months later, and to identify the kind of alerts that were dealt with. Comparison of enrolled patients on weekdays versus weekends will shed light on GPs' use of the CDSS in the absence or presence of patients. Outcomes will be presented using descriptive statistics, and significance tests will be used to identify associations between them. We will conduct subgroup analyses, including time effects to account for software improvements.
Discussion: This study protocol is the basis for conducting analyses of the quantitative process evaluation. By providing insight into how GPs conduct medication reviews, the evaluation will provide context to the trial results and support their interpretation. The evaluation relies on the proper documentation by GPs, potentially limiting its explanatory power.
Keywords: clinical decision support; polypharmacy; primary care; process evaluation; study protocol.
© The Author(s), 2022.
Conflict of interest statement
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Population Division, Department of Economic and Social Affairs, United Nations. World population ageing 2019: highlights, 2019, https://www.un.org/en/development/desa/population/publications/pdf/agein...
-
- Franchi C, Tettamanti M, Pasina L, et al.. Changes in drug prescribing to Italian community-dwelling elderly people: the EPIFARM-Elderly Project 2000-2010. Eur J Clin Pharmacol 2014; 70: 437–443. - PubMed
-
- Duerden M, Avery T, Payne R. Polypharmacy and medicines optimisation: making it safe and sound. London: The King’s Fund, 2013.
LinkOut - more resources
Full Text Sources

