Cognitive Impairment and Metabolite Profile Alterations in the Hippocampus and Cortex of Male and Female Mice Exposed to a Fat and Sugar-Rich Diet are Normalized by Diet Reversal
- PMID: 35111373
- PMCID: PMC8782561
- DOI: 10.14336/AD.2021.0720
Cognitive Impairment and Metabolite Profile Alterations in the Hippocampus and Cortex of Male and Female Mice Exposed to a Fat and Sugar-Rich Diet are Normalized by Diet Reversal
Abstract
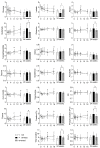
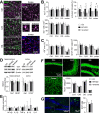
Diabetes impacts on brain metabolism, structure, and function. Alterations in brain metabolism have been observed in obesity and diabetes models induced by exposure to diets rich in saturated fat and/or sugar and have been linked to memory impairment. However, it remains to be determined whether brain dysfunction induced by obesogenic diets results from permanent brain alterations. We tested the hypothesis that an obesogenic diet (high-fat and high-sucrose diet; HFHSD) causes reversible changes in hippocampus and cortex metabolism and alterations in behavior. Mice were exposed to HFHSD for 24 weeks or for 16 weeks followed by 8 weeks of diet normalization. Development of the metabolic syndrome, changes in behavior, and brain metabolite profiles by magnetic resonance spectroscopy (MRS) were assessed longitudinally. Control mice were fed an ingredient-matched low-fat and low-sugar diet. Mice fed the HFHSD developed obesity, glucose intolerance and insulin resistance, with a more severe phenotype in male than female mice. Relative to controls, both male and female HFHSD-fed mice showed increased anxiety-like behavior, impaired memory in object recognition tasks, but preserved working spatial memory as evaluated by spontaneous alternation in a Y-maze. Alterations in the metabolite profiles were observed both in the hippocampus and cortex but were more distinct in the hippocampus. HFHSD-induced metabolic changes included altered levels of lactate, glutamate, GABA, glutathione, taurine, N-acetylaspartate, total creatine and total choline. Notably, HFHSD-induced metabolic syndrome, anxiety, memory impairment, and brain metabolic alterations recovered upon diet normalization for 8 weeks. In conclusion, cortical and hippocampal derangements induced by long-term HFHSD consumption are reversible rather than being the result of permanent tissue damage.
Keywords: anxiety; brain metabolism; diabetes; high-fat; memory; obesity; sucrose.
Copyright: © 2022 Garcia-Serrano et al.
Conflict of interest statement
Disclosure/conflict of interest The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Figures






References
-
- Askari M, Heshmati J, Shahinfar H, Tripathi N, Daneshzad E (2020). Ultra-processed food and the risk of overweight and obesity: a systematic review and meta-analysis of observational studies. Int J Obes (Lond), 44(10):2080-2091. - PubMed
-
- Frisardi V, Solfrizzi V, Seripa D, Capurso C, Santamato A, Sancarlo D, Vendemiale G, Pilotto A, Panza F (2010). Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer's disease. Ageing Res Rev, 9(4):399-417. - PubMed
LinkOut - more resources
Full Text Sources
