Development, Diversity, and Neurogenic Capacity of Enteric Glia
- PMID: 35111752
- PMCID: PMC8801887
- DOI: 10.3389/fcell.2021.775102
Development, Diversity, and Neurogenic Capacity of Enteric Glia
Abstract
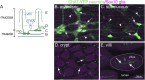
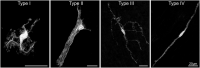
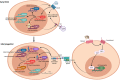
Enteric glia are a fascinating population of cells. Initially identified in the gut wall as the "support" cells of the enteric nervous system, studies over the past 20 years have unveiled a vast array of functions carried out by enteric glia. They mediate enteric nervous system signalling and play a vital role in the local regulation of gut functions. Enteric glial cells interact with other gastrointestinal cell types such as those of the epithelium and immune system to preserve homeostasis, and are perceptive to luminal content. Their functional versatility and phenotypic heterogeneity are mirrored by an extensive level of plasticity, illustrated by their reactivity in conditions associated with enteric nervous system dysfunction and disease. As one of the hallmarks of their plasticity and extending their operative relationship with enteric neurons, enteric glia also display neurogenic potential. In this review, we focus on the development of enteric glial cells, and the mechanisms behind their heterogeneity in the adult gut. In addition, we discuss what is currently known about the role of enteric glia as neural precursors in the enteric nervous system.
Keywords: enteric nervous system; glial cells; gliogenesis; neural crest; neurogenesis.
Copyright © 2022 Boesmans, Nash, Tasnády, Yang, Stamp and Hao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

