Inflammation-Induced Coagulopathy Substantially Differs Between COVID-19 and Septic Shock: A Prospective Observational Study
- PMID: 35111777
- PMCID: PMC8801505
- DOI: 10.3389/fmed.2021.780750
Inflammation-Induced Coagulopathy Substantially Differs Between COVID-19 and Septic Shock: A Prospective Observational Study
Abstract
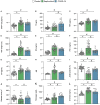
Critical COVID-19, like septic shock, is related to a dysregulated systemic inflammatory reaction and is associated with a high incidence of thrombosis and microthrombosis. Improving the understanding of the underlying pathophysiology of critical COVID-19 could help in finding new therapeutic targets already explored in the treatment of septic shock. The current study prospectively compared 48 patients with septic shock and 22 patients with critical COVID-19 regarding their clinical characteristics and outcomes, as well as key plasmatic soluble biomarkers of inflammation, coagulation, endothelial activation, platelet activation, and NETosis. Forty-eight patients with matched age, gender, and co-morbidities were used as controls. Critical COVID-19 patients exhibited less organ failure but a prolonged ICU length-of-stay due to a prolonged respiratory failure. Inflammatory reaction of critical COVID-19 was distinguished by very high levels of interleukin (IL)-1β and T lymphocyte activation (including IL-7 and CD40L), whereas septic shock displays higher levels of IL-6, IL-8, and a more significant elevation of myeloid response biomarkers, including Triggering Receptor Expressed on Myeloid cells-1 (TREM-1) and IL-1ra. Subsequent inflammation-induced coagulopathy of COVID-19 also differed from sepsis-induced coagulopathy (SIC) and was characterized by a marked increase in soluble tissue factor (TF) but less platelets, antithrombin, and fibrinogen consumption, and less fibrinolysis alteration. In conclusion, COVID-19 inflammation-induced coagulopathy substantially differs from SIC. Modulating TF release and activity should be evaluated in critical COVID-19 patients.
Keywords: COVID-19; NETosis; coagulopathy; endothelium; inflammation; platelet; septic shock.
Copyright © 2022 Dechamps, De Poortere, Martin, Gatto, Daumerie, Bouzin, Octave, Ginion, Robaux, Pirotton, Bodart, Gerard, Montiel, Campion, Gruson, Van Dievoet, Douxfils, Haguet, Morimont, Derive, Jolly, Bertrand, Dumoutier, Castanares-Zapatero, Laterre, Horman and Beauloye.
Conflict of interest statement
JD is the CEO and founder of QUALIblood s.a., a Belgian Contract Research Organization. LM and HH were employed by the company QUALIblood s.a. and authors MDer and LJ were employed by the company Inotrem s.a. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Foundation Saint-Luc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

