Computational Methods for Single-Cell Imaging and Omics Data Integration
- PMID: 35111809
- PMCID: PMC8801747
- DOI: 10.3389/fmolb.2021.768106
Computational Methods for Single-Cell Imaging and Omics Data Integration
Abstract
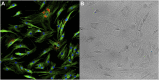
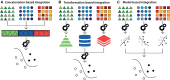
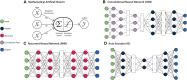
Integrating single cell omics and single cell imaging allows for a more effective characterisation of the underlying mechanisms that drive a phenotype at the tissue level, creating a comprehensive profile at the cellular level. Although the use of imaging data is well established in biomedical research, its primary application has been to observe phenotypes at the tissue or organ level, often using medical imaging techniques such as MRI, CT, and PET. These imaging technologies complement omics-based data in biomedical research because they are helpful for identifying associations between genotype and phenotype, along with functional changes occurring at the tissue level. Single cell imaging can act as an intermediary between these levels. Meanwhile new technologies continue to arrive that can be used to interrogate the genome of single cells and its related omics datasets. As these two areas, single cell imaging and single cell omics, each advance independently with the development of novel techniques, the opportunity to integrate these data types becomes more and more attractive. This review outlines some of the technologies and methods currently available for generating, processing, and analysing single-cell omics- and imaging data, and how they could be integrated to further our understanding of complex biological phenomena like ageing. We include an emphasis on machine learning algorithms because of their ability to identify complex patterns in large multidimensional data.
Keywords: ageing; data integration; machine learning; single cell imaging; single cell omics.
Copyright © 2022 Watson, Taherian Fard and Mar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Abràmoff M. D., Magalhães P. J., Ram S. J. (2004). Image Processing with ImageJ. Biophotonics Int. 11 (7), 36–42.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

