Novel nanofibrous membrane-supporting stem cell sheets for plasmid delivery and cell activation to accelerate wound healing
- PMID: 35111946
- PMCID: PMC8780893
- DOI: 10.1002/btm2.10244
Novel nanofibrous membrane-supporting stem cell sheets for plasmid delivery and cell activation to accelerate wound healing
Abstract
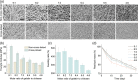
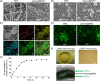
The integration of biomaterials with cells for high overall performances is vitally important in tissue engineering, as scaffold-free cell sheet lacks enough mechanical performance and cell viability while cell-free scaffold possesses limited biological functions. In this study, we propose a new strategy to strengthen cell sheets and enhance cell activity for accelerating wound healing based on a novel sandwich structure of cell sheet-plasmid@membrane-cell sheet (CpMC). Specifically, the CpMC contains two adipose-derived stem cell (ADSC) sheets on outer surfaces and an electrospun gelatin/chitosan nanofibrous membrane (NFM) encapsulating vascular endothelial growth factor (VEGF) plasmids in between. The physicochemical properties of NFM including swelling, stiffness, strength, elasticity, and biodegradation can be tailored by simply adjusting the ratio between gelatin and chitosan to be 7:3 which is optimal for most effectively supporting ADSCs adhesion and proliferation. The swelling/biodegradation of NFM mediates the sustained release of encapsulated VEGF plasmids into adjacent ADSCs, and NFM assists VEGF plasmids to promote the differentiation of ADSCs into endothelial, epidermal, and fibroblast cells, in support of the neoangiogenesis and regeneration of cutaneous tissues within 2 weeks. The proposed membrane-supporting cell sheet strategy provides a new route to tissue engineering, and the developed CpMC demonstrates a high potential for clinical translation.
Keywords: cell sheet; chitosan; gelatin; stem cell; sustained release; tissue engineering; wound healing.
© 2021 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
All authors declared no potential conflicts of interest.
Figures





Similar articles
-
Controlled pVEGF delivery via a gene-activated matrix comprised of a peptide-modified non-viral vector and a nanofibrous scaffold for skin wound healing.Acta Biomater. 2022 Mar 1;140:149-162. doi: 10.1016/j.actbio.2021.11.037. Epub 2021 Nov 28. Acta Biomater. 2022. PMID: 34852301
-
Ameliorated healing of biliary anastomosis by autologous adipose-derived stem cell sheets.Regen Ther. 2020 Jan 17;14:79-86. doi: 10.1016/j.reth.2019.11.001. eCollection 2020 Jun. Regen Ther. 2020. PMID: 31988997 Free PMC article.
-
Cryopreserved Adipose-Derived Stem Cell Sheets: An Off-the-Shelf Scaffold for Augmenting Tendon-to-Bone Healing in a Rabbit Model of Chronic Rotator Cuff Tear.Am J Sports Med. 2023 Jul;51(8):2005-2017. doi: 10.1177/03635465231171682. Epub 2023 May 25. Am J Sports Med. 2023. PMID: 37227145
-
Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing.Int J Mol Sci. 2020 Feb 14;21(4):1306. doi: 10.3390/ijms21041306. Int J Mol Sci. 2020. PMID: 32075181 Free PMC article. Review.
-
The wound-healing and antioxidant effects of adipose-derived stem cells.Expert Opin Biol Ther. 2009 Jul;9(7):879-87. doi: 10.1517/14712590903039684. Expert Opin Biol Ther. 2009. PMID: 19522555 Review.
Cited by
-
Euglena gracilis and Its Aqueous Extract Constructed With Chitosan-Hyaluronic Acid Hydrogel Facilitate Cutaneous Wound Healing in Mice Without Inducing Excessive Inflammatory Response.Front Bioeng Biotechnol. 2021 Dec 10;9:713840. doi: 10.3389/fbioe.2021.713840. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34957061 Free PMC article.
-
Controlled Thin Polydimethylsiloxane Membrane with Small and Large Micropores for Enhanced Attachment and Detachment of the Cell Sheet.Membranes (Basel). 2022 Jul 3;12(7):688. doi: 10.3390/membranes12070688. Membranes (Basel). 2022. PMID: 35877891 Free PMC article.
-
One-step fabrication of cell sheet-laden hydrogel for accelerated wound healing.Bioact Mater. 2023 Jun 20;28:420-431. doi: 10.1016/j.bioactmat.2023.06.005. eCollection 2023 Oct. Bioact Mater. 2023. PMID: 37519924 Free PMC article.
-
ISX9 loaded thermoresponsive nanoparticles for hair follicle regrowth.Mater Today Bio. 2023 Nov 3;23:100849. doi: 10.1016/j.mtbio.2023.100849. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 38033366 Free PMC article.
-
Recent Developments in Extracellular Matrix Remodeling for Fat Grafting.Front Cell Dev Biol. 2021 Dec 16;9:767362. doi: 10.3389/fcell.2021.767362. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34977018 Free PMC article. Review.
References
-
- Park KM, Shin YM, Kim K, Shin H. Tissue engineering and regenerative medicine 2017: a year in review. Tissue Eng Part B Rev. 2018;24(5):327‐344. - PubMed
-
- Baudequin T, Tabrizian M. Multilineage constructs for scaffold‐based tissue engineering: a review of tissue‐specific challenges. Adv Healthc Mater. 2018;7(3):1700734‐1700763. - PubMed
-
- Yamato M, Okano T. Cell sheet engineering. Mater Today. 2004;7(5):42‐47.
-
- Ji W, Yang F, Ma JL, et al. Incorporation of stromal cell‐derived factor‐1 alpha in PCL/gelatin electrospun membranes for guided bone regeneration. Biomaterials. 2013;34(3):735‐745. - PubMed
LinkOut - more resources
Full Text Sources

