Effect of continuing versus stopping pre-stroke antihypertensive agents within 12 h on outcome after stroke: A subgroup analysis of the efficacy of nitric oxide in stroke (ENOS) trial
- PMID: 35112073
- PMCID: PMC8790472
- DOI: 10.1016/j.eclinm.2022.101274
Effect of continuing versus stopping pre-stroke antihypertensive agents within 12 h on outcome after stroke: A subgroup analysis of the efficacy of nitric oxide in stroke (ENOS) trial
Abstract
Background: It is not known whether to continue or temporarily stop existing antihypertensive drugs in patients with acute stroke.
Methods: We performed a prospective subgroup analysis of patients enrolled into the Efficacy of Nitric Oxide in Stroke (ENOS) trial who were randomised to continue vs stop prior antihypertensive therapy within 12 h of stroke onset. The primary outcome was functional outcome, assessed with the modified Rankin Scale at 90 days by observers blinded to treatment assignment, and analysed with ordinal logistic regression.
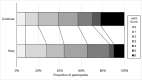
Findings: Of 4011 patients recruited into ENOS from 2001 to 2014, 2097 patients were randomised to continue vs stop prior antihypertensive treatment, and 384 (18.3%, continue 185, stop 199) were enrolled within 12 h of ictus: mean (SD) age 71.8 (11.8) years, female 193 (50.3%), ischaemic stroke 342 (89.1%) and total anterior circulation syndrome 114 (29.7%). As compared with stopping, continuing treatment within 12 h of onset lowered blood pressure by 15.5/9.6 mmHg (p<0.001/<0.001) by 7 days, shifted the modified Rankin Scale to a worse outcome by day 90, adjusted common odds ratio (OR) 1.46 (95% CI 1.01-2.11), and was associated with an increased death rate by day 90 (hazard ratio 2.17, 95% CI 1.24-3.79). Other outcomes (disability - Barthel Index, quality of life - EQ-visual analogue scale, cognition - telephone mini-mental state examination, and mood - Zung depression scale) were also worse with continuing treatment.
Interpretation: In this pre-specified subgroup analysis of the large ENOS trial, continuing prior antihypertensive therapy within 12 h of stroke onset in a predominantly ischaemic stroke population was unsafe with worse functional outcome, disability, cognition, mood, quality of life and increased death. Future studies assessing continuing or stopping prior antihypertensives in the context of thrombectomy are awaited.
© 2022 The Authors.
Conflict of interest statement
JPA was supported in part by National Institute of Health Research (NIHR) TARDIS (10/104/24) and British Heart Foundation RIGHT-2 (CS/14/4/30972) and is supported by a NIHR Health and Care Research Scholarship. JMW was supported, in part, by the Scottish Funding Council through the SINAPSE Collaboration and the UK Dementia Research Institute which receives funding from DRI Ltd, funded by the UK Medical Research Council, Alzheimer's Society and Alzheimer's Research UK. PMB is Stroke Association Professor of Stroke Medicine and a NIHR Senior Investigator. All other authors report no declarations.
Figures


References
-
- Neal B., MacMahon S., Chapman N. Blood pressure lowering treatment trialists C. effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. blood pressure lowering treatment trialists' collaboration. Lancet. 2000;356(9246):1955–1964. - PubMed
-
- Rashid P., Leonardi-Bee J., Bath P. Blood pressure reduction and the secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741–2749. - PubMed
-
- Lees K.R., Dyker A.G. Blood pressure control after acute stroke. J Hypertens. 1996;14(suppl 6):S35–SS8. - PubMed
-
- Bath P.M.W., Weaver C., Iddenden R., Bath F.J. A trial of blood pressure reduction in acute stroke. Age Ageing. 2000;29:554–555. - PubMed
-
- Royal College of Physicians . National Clinical Guidelines for Stroke. 5th ed. Royal College of Physicians; 2016. Intercollegiate stroke working party.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

