C-reactive protein levels and risk of dementia-Observational and genetic studies of 111,242 individuals from the general population
- PMID: 35112776
- PMCID: PMC9790296
- DOI: 10.1002/alz.12568
C-reactive protein levels and risk of dementia-Observational and genetic studies of 111,242 individuals from the general population
Abstract
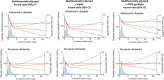
Introduction: Increased plasma levels of C-reactive protein (CRP) in midlife are associated with increased risk of Alzheimer's disease (AD), whereas in older age the opposite association is observed. Whether genetically determined CRP is associated with AD remains unclear.
Methods: A total of 111,242 White individuals from the Copenhagen General Population Study and the Copenhagen City Heart Study were included. Plasma levels of CRP and four regulatory genetic variants in the CRP gene were determined.
Results: For CRP percentile group 1 to 5 (lowest plasma CRP) versus the 50 to 75 group (reference), the hazard ratio for AD was 1.69 (95% confidence interval 1.29-2.16). Genetically low CRP was associated with increased risk of AD in individuals with body mass index ≤25 kg/m2 (P = 4 × 10-6 ).
Discussion: Low plasma levels of CRP at baseline were associated with high risk of AD in individuals from the general population. These observational findings were supported by genetic studies.
Keywords: Alzheimer's disease; C-reactive protein; CRP; body mass index; gene-environment interaction.
© 2022 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
SHH, JQT, IJR, and ATH have nothing to disclose. BGN received consulting fees (personal fees) from AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, Silence Therapeutics. RFS received consulting fees (personal fees) from Novo Nordisk.
Figures


Comment in
-
Reply to "Mendelian randomization highlights causal association between genetically increased C-reactive protein levels and reduced Alzheimer's disease risk".Alzheimers Dement. 2022 Oct;18(10):2007-2009. doi: 10.1002/alz.12688. Epub 2022 May 22. Alzheimers Dement. 2022. PMID: 35598326 No abstract available.
-
Mendelian randomization highlights causal association between genetically increased C-reactive protein levels and reduced Alzheimer's disease risk.Alzheimers Dement. 2022 Oct;18(10):2003-2006. doi: 10.1002/alz.12687. Epub 2022 May 22. Alzheimers Dement. 2022. PMID: 35598332 No abstract available.
-
Observational and genetic studies of C-reactive protein levels and risk of Alzheimer's disease.Alzheimers Dement. 2022 Dec;18(12):2734-2735. doi: 10.1002/alz.12742. Epub 2022 Aug 21. Alzheimers Dement. 2022. PMID: 35988061 No abstract available.
References
-
- Prince PM, Wimo A, Guerchet MM, Ali GC, Wu YT, Prina M, World Alzheimer Report 2015 ‐ the global impact of dementia. An analysis of prevalence, incidence, and cost and trends. London: Alzheimer's Disease International; 2015:84.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

