COVID-19 vaccine effectiveness among immunocompromised populations: a targeted literature review of real-world studies
- PMID: 35112973
- PMCID: PMC8862165
- DOI: 10.1080/14760584.2022.2035222
COVID-19 vaccine effectiveness among immunocompromised populations: a targeted literature review of real-world studies
Abstract
Introduction: From July through October of 2021, several countries issued recommendations for increased COVID-19 vaccine protection for individuals with one or more immunocompromised (IC) conditions. It is critically important to understand the vaccine effectiveness (VE) of COVID-19 vaccines among IC populations as recommendations are updated over time in response to the evolving COVID-19 pandemic.
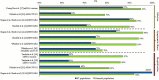
Areas covered: A targeted literature review was conducted to identify real-world studies that assessed COVID-19 VE in IC populations between December 2020 and September 2021. A total of 10 studies from four countries were identified and summarized in this review.
Expert opinion: VE of the widely available COVID-19 vaccines, including BNT162b2 (Pfizer/BioNTech), mRNA-1273 (Moderna), Ad26.COV2.S (Janssen), and ChAdOx1 nCoV-19 (Oxford/AstraZeneca), ranged from 64% to 90% against SARS-CoV-2 infection, 73% to 84% against symptomatic illness, 70% to 100% against severe illness, and 63% to 100% against COVID-19-related hospitalization among the fully vaccinated IC populations included in the studies. COVID-19 VE for most outcomes in the IC populations included in these studies were lower than in the general populations. These findings provide preliminary evidence that the IC population requires greater protective measures to prevent COVID-19 infection and associated illness, hence should be prioritized while implementing recommendations of additional COVID-19 vaccine doses.
Keywords: COVID-19 vaccines; COVID-19-related hospitalization; SARS-CoV-2 infection; immunocompromised; symptomatic COVID-19 illness; vaccine effectiveness.
Figures
References
-
- OurWorldinData.org [Internet] . Statistics and research: coronavirus (COVID-19) vaccinations. London (England): Oxford; [cited 2021 Sep 30]. Available from: https://ourworldindata.org/covid-vaccinations
-
- Cdc.gov [Internet] . Atlanta (GA): centers for disease control and prevention. Science brief: COVID-19 vaccines and vaccination. Updated 2021 Sep 15. cited 2021 Oct 19]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/fully-v.... - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous