Association of Pegcetacoplan With Progression of Incomplete Retinal Pigment Epithelium and Outer Retinal Atrophy in Age-Related Macular Degeneration: A Post Hoc Analysis of the FILLY Randomized Clinical Trial
- PMID: 35113137
- PMCID: PMC8814977
- DOI: 10.1001/jamaophthalmol.2021.6067
Association of Pegcetacoplan With Progression of Incomplete Retinal Pigment Epithelium and Outer Retinal Atrophy in Age-Related Macular Degeneration: A Post Hoc Analysis of the FILLY Randomized Clinical Trial
Abstract
Importance: Change in areas of incomplete retinal pigment epithelium (RPE) and outer retinal atrophy (iRORA) within eyes with geographic atrophy (GA) might reflect similar changes among eyes with drusen but no GA.
Objective: To evaluate the potential association of pegcetacoplan with progression of iRORA in eyes with GA secondary to AMD.
Design, setting, and participants: This post hoc analysis of the phase 2 multicenter, randomized, single-masked, sham-controlled FILLY trial of intravitreal pegcetacoplan for 12 months took place from February 2 to July 7, 2020. Participants comprised 167 patients with GA secondary to AMD who received pegcetacoplan monthly (n = 41) or every other month (n = 56) or a sham injection (n = 70) in the FILLY trial, completed the month 12 study visit, and did not develop exudative AMD.
Interventions: Intravitreal pegcetacoplan, 15 mg, or sham injection, monthly or every other month for 12 months.
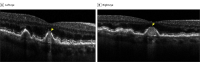
Main outcomes and measures: Masked readers analyzed spectral-domain optical coherence tomography scans in regions beyond a perimeter of 500 μm from the GA border according to the Classification of Atrophy Meetings criteria. Primary outcome measures were progression from iRORA to complete RPE and outer retina atrophy (cRORA) from baseline to 6 and 12 months.
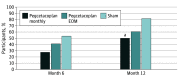
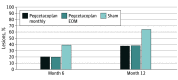
Results: Among the 167 patients in the study, at baseline, iRORA was present in 45.0% of study eyes (18 of 40) in the pegcetacoplan monthly group, 61.8% of study eyes (34 of 55) in the pegcetacoplan every other month group, and 50.7% of study eyes (34 of 67) in the sham group. At 12 months, progression from iRORA to cRORA occurred in 50.0% of study eyes (9 of 18) in the pegcetacoplan monthly group (P = .02 vs sham), 60.6% of study eyes (20 of 33) in the pegcetacoplan every other month group (P = .06 vs sham), and 81.8% of study eyes (27 of 33) in the sham group. Compared with sham treatment, the relative risk of progression at 12 months from iRORA to cRORA was 0.61 (95% CI, 0.37-1.00) for eyes in the pegcetacoplan monthly group and 0.74 (95% CI, 0.54-1.02) for eyes in the pegcetacoplan every other month group.
Conclusions and relevance: Eyes receiving intravitreal pegcetacoplan had lower rates of progression from iRORA to cRORA compared with controls, suggesting a potential role for pegcetacoplan therapy earlier in the progression of AMD prior to the development of GA.
Trial registration: ClinicalTrials.gov Identifier: NCT02503332.
Conflict of interest statement
Figures


Comment in
-
Complement Inhibition in Age-Related Macular Degeneration-Treat Early!JAMA Ophthalmol. 2022 Mar 1;140(3):250-251. doi: 10.1001/jamaophthalmol.2021.6068. JAMA Ophthalmol. 2022. PMID: 35113132 No abstract available.
References
-
- Rahimy E, Khan MA, Chao W, Ribeiro R, Ho A, Holekamp N. Evaluation of geographic atrophy (GA) secondary to AMD in real-world clinical practice: analysis of the AAO IRIS registry. Presented at the American Academy of Ophthalmology Annual Meeting; November 13, 2020; virtual.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

