Bridged Piperidine Analogues of a High Affinity Naphthalene-Based P2Y14R Antagonist
- PMID: 35113556
- PMCID: PMC8881401
- DOI: 10.1021/acs.jmedchem.1c01964
Bridged Piperidine Analogues of a High Affinity Naphthalene-Based P2Y14R Antagonist
Abstract
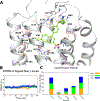
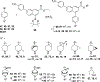
High affinity phenyl-piperidine P2Y14R antagonist 1 (PPTN) was modified with piperidine bridging moieties to probe receptor affinity and hydrophobicity. Various 2-azanorbornane, nortropane, isonortropane, isoquinuclidine, and ring-opened cyclopentylamino derivatives preserved human P2Y14R affinity (fluorescence binding assay), and their pharmacophoric overlay was compared. Enantiomeric 2-azabicyclo[2.2.1]hept-5-en-3-one precursors assured stereochemically unambiguous, diverse products. Pure (S,S,S) 2-azanorbornane enantiomer 15 (MRS4738) displayed higher affinity than 1 (3-fold higher affinity than enantiomer 16) and in vivo antihyperallodynic and antiasthmatic activity. Its double prodrug 143 (MRS4815) dramatically reduced lung inflammation in a mouse asthma model. Related lactams 21-24 and dicarboxylate 42 displayed intermediate affinity and enhanced aqueous solubility. Isoquinuclidine 34 (IC50 15.6 nM) and isonortropanol 30 (IC50 21.3 nM) had lower lipophilicity than 1. In general, rigidified piperidine derivatives did not lower lipophilicity dramatically, except those rings with multiple polar groups. P2Y14R molecular modeling based on a P2Y12R structure showed stable and persistent key interactions for compound 15.
Figures





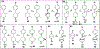


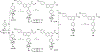

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

