The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review
- PMID: 35113777
- PMCID: PMC8862168
- DOI: 10.1080/21645515.2022.2027160
The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review
Abstract
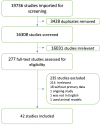
With the relatively rapid development of the COVID-19 pandemic, vaccine development has become crucial for limiting disease transmission. The accelerated growth in the approved COVID-19 vaccines has sparked concerns about their efficacies which have been assessed by many studies. This systematic review compares the efficacy and effectiveness of seven COVID-19 vaccines. A comprehensive systematic literature search was performed using several databases to identify studies reporting the effectiveness or the efficacy of the vaccines. Only 42 studies met our inclusion criteria, which revealed that the COVID-19 vaccines have successfully reduced the rates of infections, severity, hospitalization, and mortality among the different populations. The full-dose regimen of the Pfizer/BioNTech vaccine is the most effective against infections with the B.1.1.7 and B.1.351 variants. Despite of the high effectiveness of some of the COVID-19 vaccines, more efforts are required to test their effectiveness against the other newly emerging variants.
Keywords: COVID-19; effectiveness; efficacy; vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- COVID-19 Map [Internet] . Johns Hopkins coronavirus resource center [cited 2021 Jun 2]. Available from: https://coronavirus.jhu.edu/map.html.
-
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB.. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA [Internet]. 2020. [cited 2021 Sep 7]. https://jamanetwork.com/journals/jama/fullarticle/2764727. - PubMed
-
- García-Montero C, Fraile-Martínez O, Bravo C, Torres-Carranza D, Sanchez-Trujillo L, Gómez-Lahoz AM, Guijarro LG, García-Honduvilla N, Asúnsolo A, Bujan J, et al. An updated review of sars-cov-2 vaccines and the importance of effective vaccination programs in pandemic times. Vaccines. 2021;9:433.5. doi: 10.3390/vaccines9050433. - DOI - PMC - PubMed
-
- McGill COVID-19 vaccine tracker [Internet]. [cited 2021 June 5]. https://covid19.trackvaccines.org/.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous