Targeting TGF-β for treatment of osteogenesis imperfecta
- PMID: 35113812
- PMCID: PMC8970679
- DOI: 10.1172/JCI152571
Targeting TGF-β for treatment of osteogenesis imperfecta
Abstract
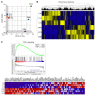
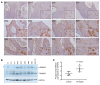
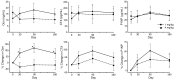
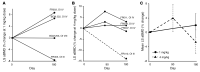
BACKGROUNDCurrently, there is no disease-specific therapy for osteogenesis imperfecta (OI). Preclinical studies demonstrate that excessive TGF-β signaling is a pathogenic mechanism in OI. Here, we evaluated TGF-β signaling in children with OI and conducted a phase I clinical trial of TGF-β inhibition in adults with OI.METHODSHistology and RNA-Seq were performed on bones obtained from children. Gene Ontology (GO) enrichment assay, gene set enrichment analysis (GSEA), and Ingenuity Pathway Analysis (IPA) were used to identify dysregulated pathways. Reverse-phase protein array, Western blot, and IHC were performed to evaluate protein expression. A phase I study of fresolimumab, a TGF-β neutralizing antibody, was conducted in 8 adults with OI. Safety and effects on bone remodeling markers and lumbar spine areal bone mineral density (LS aBMD) were assessed.RESULTSOI bone demonstrated woven structure, increased osteocytes, high turnover, and reduced maturation. SMAD phosphorylation was the most significantly upregulated GO molecular event. GSEA identified the TGF-β pathway as the top activated signaling pathway, and IPA showed that TGF-β1 was the most significant activated upstream regulator mediating the global changes identified in OI bone. Treatment with fresolimumab was well-tolerated and associated with increases in LS aBMD in participants with OI type IV, whereas participants with OI type III and VIII had unchanged or decreased LS aBMD.CONCLUSIONIncreased TGF-β signaling is a driver pathogenic mechanism in OI. Anti-TGF-β therapy could be a potential disease-specific therapy, with dose-dependent effects on bone mass and turnover.TRIAL REGISTRATIONClinicalTrials.gov NCT03064074.FUNDINGBrittle Bone Disorders Consortium (U54AR068069), Clinical Translational Core of Baylor College of Medicine Intellectual and Developmental Disabilities Research Center (P50HD103555) from National Institute of Child Health and Human Development, USDA/ARS (cooperative agreement 58-6250-6-001), and Sanofi Genzyme.
Keywords: Bone disease; Clinical Trials; Drug therapy; Therapeutics.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

