Arginine deprivation alters microglial polarity and synergizes with radiation to eradicate non-arginine-auxotrophic glioblastoma tumors
- PMID: 35113813
- PMCID: PMC8920336
- DOI: 10.1172/JCI142137
Arginine deprivation alters microglial polarity and synergizes with radiation to eradicate non-arginine-auxotrophic glioblastoma tumors
Abstract
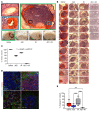
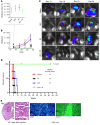
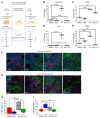
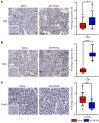
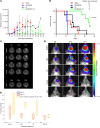
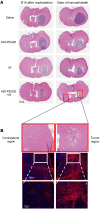
New approaches for the management of glioblastoma (GBM) are an urgent and unmet clinical need. Here, we illustrate that the efficacy of radiotherapy for GBM is strikingly potentiated by concomitant therapy with the arginine-depleting agent ADI-PEG20 in a non-arginine-auxotrophic cellular background (argininosuccinate synthetase 1 positive). Moreover, this combination led to durable and complete radiological and pathological response, with extended disease-free survival in an orthotopic immune-competent model of GBM, with no significant toxicity. ADI-PEG20 not only enhanced the cellular sensitivity of argininosuccinate synthetase 1-positive GBM to ionizing radiation by elevated production of nitric oxide (˙NO) and hence generation of cytotoxic peroxynitrites, but also promoted glioma-associated macrophage/microglial infiltration into tumors and turned their classical antiinflammatory (protumor) phenotype into a proinflammatory (antitumor) phenotype. Our results provide an effective, well-tolerated, and simple strategy to improve GBM treatment that merits consideration for early evaluation in clinical trials.
Keywords: Amino acid metabolism; Brain cancer; Nitric oxide; Oncology; Therapeutics.
Conflict of interest statement
Figures