On-line Electrode Dissolution Monitoring during Organic Electrosynthesis: Direct Evidence of Electrode Dissolution during Kolbe Electrolysis
- PMID: 35114080
- PMCID: PMC9304240
- DOI: 10.1002/cssc.202102228
On-line Electrode Dissolution Monitoring during Organic Electrosynthesis: Direct Evidence of Electrode Dissolution during Kolbe Electrolysis
Abstract
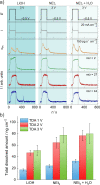
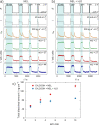
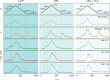
Electrode dissolution was monitored in real-time during Kolbe electrolysis along with the characteristic products. The fast determination of appropriate reaction conditions in electro-organic chemistry enables the minimization of electrode degradation while keeping an eye on the optimal formation rate and distribution of products. Herein, essential parameters influencing the dissolution of the electrode material platinum in a Kolbe electrolysis were pinpointed. The formation of reaction products and soluble platinum species were monitored during potentiodynamic and potentiostatic experiments using an electroanalytical flow cell coupled to two different mass spectrometers. The approach opens new vistas in the field of electro-organic chemistry because it enables precise and quick quantification of dissolved metals during electrosynthesis, also involving electrode materials other than platinum. Furthermore, it draws attention to the vital topic of electrode stability in electro-organic synthesis, which becomes increasingly important for the implementation of green chemical processes utilizing renewable energy.
Keywords: Kolbe electrolysis; Pt dissolution; electrochemistry; electrode stability; online electrochemical mass spectrometer.
© 2022 The Authors. ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Dissolution of platinum: limits for the deployment of electrochemical energy conversion?Angew Chem Int Ed Engl. 2012 Dec 7;51(50):12613-5. doi: 10.1002/anie.201207256. Epub 2012 Nov 4. Angew Chem Int Ed Engl. 2012. PMID: 23124819 Free PMC article.
-
Online monitoring of electrocatalytic reactions of alcohols at platinum and gold electrodes in acidic, neutral and alkaline media by capillary electrophoresis with contactless conductivity detection (EC-CE-C4 D).Electrophoresis. 2017 Nov;38(21):2725-2732. doi: 10.1002/elps.201700124. Epub 2017 May 26. Electrophoresis. 2017. PMID: 28485016
-
Beyond Kolbe and Hofer-Moest: Electrochemical Synthesis of Carboxylic Anhydrides from Carboxylic Acids.ChemistryOpen. 2022 May;11(5):e202200059. doi: 10.1002/open.202200059. ChemistryOpen. 2022. PMID: 35561027 Free PMC article.
-
Deciphering platinum dissolution in neural stimulation electrodes: Electrochemistry or biology?Biomaterials. 2024 Sep;309:122575. doi: 10.1016/j.biomaterials.2024.122575. Epub 2024 Apr 17. Biomaterials. 2024. PMID: 38677220 Review.
-
Bioinspired Electrolysis for Green Molecular Transformations of Organic Halides Catalyzed by B12 Complex.Chem Rec. 2021 Sep;21(9):2080-2094. doi: 10.1002/tcr.202100077. Epub 2021 Jun 1. Chem Rec. 2021. PMID: 34075694 Review.
Cited by
-
Anion intercalation enables efficient and stable carboxylate upgrading via aqueous non-Kolbe electrolysis.Nat Commun. 2025 Apr 19;16(1):3719. doi: 10.1038/s41467-025-58924-x. Nat Commun. 2025. PMID: 40253422 Free PMC article.
References
-
- None
-
- Frontana-Uribe B. A., Little R. D., Ibanez J. G., Palma A., Vasquez-Medrano R., Green Chem. 2010, 12, 2099–2119;
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

