A Selective and Orally Bioavailable Quinoline-6-Carbonitrile-Based Inhibitor of CDK8/19 Mediator Kinase with Tumor-Enriched Pharmacokinetics
- PMID: 35114084
- PMCID: PMC10042267
- DOI: 10.1021/acs.jmedchem.1c01951
A Selective and Orally Bioavailable Quinoline-6-Carbonitrile-Based Inhibitor of CDK8/19 Mediator Kinase with Tumor-Enriched Pharmacokinetics
Abstract
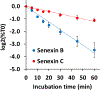
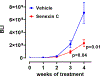
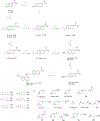
Senexins are potent and selective quinazoline inhibitors of CDK8/19 Mediator kinases. To improve their potency and metabolic stability, quinoline-based derivatives were designed through a structure-guided strategy based on the simulated drug-target docking model of Senexin A and Senexin B. A library of quinoline-Senexin derivatives was synthesized to explore the structure-activity relationship (SAR). An optimized compound 20a (Senexin C) exhibits potent CDK8/19 inhibitory activity with high selectivity. Senexin C is more metabolically stable and provides a more sustained inhibition of CDK8/19-dependent cellular gene expression when compared with the prototype inhibitor Senexin B. In vivo pharmacokinetic (PK) and pharmacodynamic (PD) evaluation using a novel tumor-based PD assay showed good oral bioavailability of Senexin C with a strong tumor-enrichment PK profile and tumor-PD marker responses. Senexin C inhibits MV4-11 leukemia growth in a systemic in vivo model with good tolerability.
Conflict of interest statement
The authors declare the following competing financial interest(s):
D.C.P, M.C. and I.B.R. are or have been employees or officers, and J.L. is a consultant of Senex Biotechnology, Inc. A.M. and S.G. are or have been employees or officers of CSC BIOCAD.
Figures
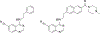
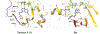
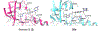

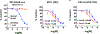
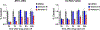


References
-
- Liu Y; Ranish JA; Aebersold R; Hahn S Yeast nuclear extract contains two major forms of RNA polymerase II mediator complexes. Journal of Biological Chemistry 2001, 276, 7169–7175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials
Miscellaneous

