Therapeutic Potential of HNF4α in End-stage Liver Disease
- PMID: 35114889
- PMCID: PMC9208774
- DOI: 10.1080/15476278.2021.1994273
Therapeutic Potential of HNF4α in End-stage Liver Disease
Abstract
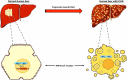
The prevalence of end-stage liver disease (ESLD) in the US is increasing at an alarming rate. It can be caused by several factors; however, one of the most common routes begins with nonalcoholic fatty liver disease (NAFLD). ESLD is diagnosed by the presence of irreversible damage to the liver. Currently, the only definitive treatment for ESLD is orthotopic liver transplantation (OLT). Nevertheless, OLT is limited due to a shortage of donor livers. Several promising alternative treatment options are under investigation. Researchers have focused on the effect of liver-enriched transcription factors (LETFs) on disease progression. Specifically, hepatocyte nuclear factor 4-alpha (HNF4α) has been reported to reset the liver transcription network and possibly play a role in the regression of fibrosis and cirrhosis. In this review, we describe the function of HNF4α, along with its regulation at various levels. In addition, we summarize the role of HNF4α in ESLD and its potential as a therapeutic target in the treatment of ESLD.
Keywords: ESLD; HNF4α; LETFs; hepatocytes; molecular therapy.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical