Phase I interim results of a phase I/II study of the IgG-Fc fusion COVID-19 subunit vaccine, AKS-452
- PMID: 35115195
- PMCID: PMC8802018
- DOI: 10.1016/j.vaccine.2022.01.043
Phase I interim results of a phase I/II study of the IgG-Fc fusion COVID-19 subunit vaccine, AKS-452
Abstract
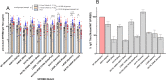
To address the coronavirus disease 2019 (COVID-19) pandemic caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a recombinant subunit vaccine, AKS-452, is being developed comprising an Fc fusion protein of the SARS-CoV-2 viral spike protein receptor binding domain (SP/RBD) antigen and human IgG1 Fc emulsified in the water-in-oil adjuvant, Montanide™ ISA 720. A single-center, open-label, phase I dose-finding and safety study was conducted with 60 healthy adults (18-65 years) receiving one or two doses 28 days apart of 22.5 µg, 45 µg, or 90 µg of AKS-452 (i.e., six cohorts, N = 10 subjects per cohort). Primary endpoints were safety and reactogenicity and secondary endpoints were immunogenicity assessments. No AEs ≥ 3, no SAEs attributable to AKS-452, and no SARS-CoV-2 viral infections occurred during the study. Seroconversion rates of anti-SARS-CoV-2 SP/RBD IgG titers in the 22.5, 45, and 90 µg cohorts at day 28 were 70%, 90%, and 100%, respectively, which all increased to 100% at day 56 (except 89% for the single-dose 22.5 µg cohort). All IgG titers were Th1-isotype skewed and efficiently bound mutant SP/RBD from several SARS-CoV-2 variants with strong neutralization potencies of live virus infection of cells (including alpha and delta variants). The favorable safety and immunogenicity profiles of this phase I study (ClinicalTrials.gov: NCT04681092) support phase II initiation of this room-temperature stable vaccine that can be rapidly and inexpensively manufactured to serve vaccination at a global scale without the need of a complex distribution or cold chain.
Keywords: AKS-452; COVID-19; Coronavirus; Fc-fusion; Infectious disease; Pandemic; Prophylaxis; SARS-CoV-2; Vaccine.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The following authors declare the following financial interests/personal relationships which may be considered as potential competing interests: the following authors were employed by and received monetary compensation from Akston Biosciences, Inc.: DGA, ARD, MMS, SM, RR, EKG, TS, SK, JRH, NJS, VR, SN, TML, TZ. No other authors were personally compensated by Akston Biosciences.
Figures



References
-
- University JH. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University;https://www.covidtracker.com/.
-
- Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–1993. - PMC - PubMed
-
- Zhu F.-C., Guan X.-H., Li Y.-H., Huang J.-Y., Jiang T., Hou L.-H., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

