Comprehensive Predictions of Cytochrome P450 (P450)-Mediated In Vivo Cannabinoid-Drug Interactions Based on Reversible and Time-Dependent P450 Inhibition in Human Liver Microsomes
- PMID: 35115300
- PMCID: PMC11022902
- DOI: 10.1124/dmd.121.000734
Comprehensive Predictions of Cytochrome P450 (P450)-Mediated In Vivo Cannabinoid-Drug Interactions Based on Reversible and Time-Dependent P450 Inhibition in Human Liver Microsomes
Abstract
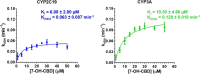
We previously reported the unbound reversible (IC50,u) and time-dependent (KI,u) inhibition potencies of cannabidiol (CBD), delta-9-tetrahydrocannabinol (THC), and THC metabolites 11-hydroxy THC (11-OH THC) and 11-nor-9-carboxy-delta-9-THC (11-COOH THC) against the major cytochrome P450 (P450) enzymes (1A2, 2C9, 2C19, 2D6, and 3A). Here, using human liver microsomes, we determined the CYP2A6, 2B6, and 2C8 IC50,u values of the aforementioned cannabinoids and the IC50,u and KI,u of the circulating CBD metabolites 7-hydroxy CBD (7-OH CBD) and 7-carboxy CBD (7-COOH CBD), against all the P450s listed above. The IC50,u of CBD, 7-OH CBD, THC, and 11-OH THC against CYP2B6 was 0.05, 0.34, 0.40, and 0.32 μM, respectively, and against CYP2C8 was 0.28, 1.02, 0.67, and 3.66 μM, respectively. 7-COOH CBD, but not 11-COOH THC, was a weak inhibitor of CYP2B6 and 2C8. All tested cannabinoids except 11-COOH THC were weak inhibitors of CYP2A6. 7-OH CBD inhibited all P450s examined (IC50,u<2.5 μM) except CYP1A2 and inactivated CYP2C19 and CYP3A, with inactivation efficiencies (kinact/KI,u) of 0.10 and 0.14 minutes-1 μM-1, respectively. Using several different static models, we predicted the following maximum pharmacokinetic interactions (affected P450 probe drug and area under the plasma concentration-time curve ratio) between oral CBD (700 mg) and drugs predominantly metabolized by CYP3A (midazolam, 14.8) > 2C9 (diclofenac, 9.6) > 2C19 (omeprazole, 7.3) > 1A2 (theophylline, 4.0) > 2B6 (ticlopidine, 2.2) > 2D6 (dextromethorphan, 2.1) > 2C8 (repaglinide, 1.6). Oral (130 mg) or inhaled (75 mg) THC was predicted to precipitate interactions with drugs predominately metabolized by CYP2C9 (diclofenac, 6.6 or 2.3, respectively) > 3A (midazolam, 1.8) > 1A2 (theophylline, 1.4). In vivo drug interaction studies are warranted to verify these predictions. SIGNIFICANCE STATEMENT: This study, combined with our previous findings, provides for the first time a comprehensive analysis of the potential for cannabidiol, delta-9-tetrahydrocannabinol, and their metabolites to inhibit cytochrome P450 enzymes in a reversible or time-dependent manner. These analyses enabled us to predict the potential of these cannabinoids to produce drug interactions in vivo at clinical or recreational doses.
Copyright © 2022 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Amaral Silva D, Pate DW, Clark RD, Davies NM, El-Kadi AOS, Löbenberg R (2020) Phytocannabinoid drug-drug interactions and their clinical implications. Pharmacol Ther 215:107621. - PubMed
-
- Anderson LL, Doohan PT, Oldfield L, Kevin RC, Arnold JC, Berger M, Amminger GP, McGregor IS (2021) Citalopram and cannabidiol: in vitro and in vivo evidence of pharmacokinetic interactions relevant to the treatment of anxiety disorders in young people. J Clin Psychopharmacol 41:525–533. - PubMed
-
- Bansal S, Paine M, Unadkat J (2021) Can cannabinoids precipitate UGT-mediated drug interactions? FASEB J 35:03854.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

