EPOS trial: the effect of air filtration through a plasma chamber on the incidence of surgical site infection in orthopaedic surgery: a study protocol of a randomised, double-blind, placebo-controlled trial
- PMID: 35115346
- PMCID: PMC8814745
- DOI: 10.1136/bmjopen-2020-047500
EPOS trial: the effect of air filtration through a plasma chamber on the incidence of surgical site infection in orthopaedic surgery: a study protocol of a randomised, double-blind, placebo-controlled trial
Abstract
Introduction: There is controversy regarding the importance of air-transmitted infections for surgical site infections (SSIs) after orthopaedic surgery. Research has been hindered by both the inability in blinding the exposure, and by the need for recruiting large enough cohorts. The aim of this study is to investigate whether using a new form of air purifier using plasma air purification (PAP) in operating rooms (ORs) lowers the SSI rate or not.
Methods and analysis: Multicentre, double-blind, cluster-randomised, placebo-controlled trial conducted at seven hospitals in 2017-2022. All patients that undergo orthopaedic surgery for minimum 30 min are included. Intervention group: patients operated in OR with PAP devices turned on.
Control group: patients operated in OR with PAP devices turned off. Randomisation: each OR will be randomised in periods of 4 weeks, 6 weeks or 8 weeks to either have the devices on or off.
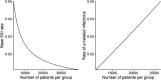
Primary outcome: any SSI postoperatively defined as a composite endpoint of any of the following: use of isoxazolylpenicillin, clindamycin or rifampicin for 2 days or more, International Classification of Diseases codes or Nordic Medico-Statistical Committee codes indicating postoperative infection. In a second step, we will perform a chart review on those patients with positive indicators of SSI to further validate the outcome. Secondary outcomes are described in the Methods section. Power: we assume an SSI rate of 2%, an SSI reduction rate of 25% and we need approximately 45 000 patients to attain a power of 80% at a significance level of 0.05.
Ethics and dissemination: The study is approved by the Swedish Ethical Review Authority. The interim analysis results from the study will be presented only to the researchers involved unless the study thereafter is interrupted for whatever reason. Publication in a medical journal will be presented after inclusion of the last patient.
Trial registration number: NCT02695368.
Keywords: adult orthopaedics; hip; infection control; knee; orthopaedic & trauma surgery.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Multicentre randomised double-blind placebo controlled trial of combination vancomycin and cefazolin surgical antibiotic prophylaxis: the Australian surgical antibiotic prophylaxis (ASAP) trial.BMJ Open. 2019 Nov 3;9(11):e033718. doi: 10.1136/bmjopen-2019-033718. BMJ Open. 2019. PMID: 31685516 Free PMC article.
-
Effect of Plasma Air Purifiers on Infection Rates in Orthopedic Surgery.NEJM Evid. 2025 Apr;4(4):EVIDoa2400289. doi: 10.1056/EVIDoa2400289. Epub 2025 Mar 25. NEJM Evid. 2025. PMID: 40130977 Clinical Trial.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Is the Risk of Infection Lower with Sutures than with Staples for Skin Closure After Orthopaedic Surgery? A Meta-analysis of Randomized Trials.Clin Orthop Relat Res. 2019 May;477(5):922-937. doi: 10.1097/CORR.0000000000000690. Clin Orthop Relat Res. 2019. PMID: 30958392 Free PMC article.
-
Laminar airflow ventilation systems in orthopaedic operating room do not prevent surgical site infections: a systematic review and meta-analysis.J Orthop Surg Res. 2023 Aug 5;18(1):572. doi: 10.1186/s13018-023-03992-2. J Orthop Surg Res. 2023. PMID: 37543643 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials