Optimising Access Surgery in Senior Haemodialysis Patients (OASIS): study protocol for a multicentre randomised controlled trial
- PMID: 35115352
- PMCID: PMC8814743
- DOI: 10.1136/bmjopen-2021-053108
Optimising Access Surgery in Senior Haemodialysis Patients (OASIS): study protocol for a multicentre randomised controlled trial
Abstract
Introduction: Current evidence on vascular access strategies for haemodialysis patients is based on observational studies that are at high risk of selection bias. For elderly patients, autologous arteriovenous fistulas that are typically created in usual care may not be the best option because a significant proportion of fistulas either fail to mature or remain unused. In addition, long-term complications associated with arteriovenous grafts and central venous catheters may be less relevant when considering the limited life expectancy of these patients. Therefore, we designed the Optimising Access Surgery in Senior Haemodialysis Patients (OASIS) trial to determine the best strategy for vascular access creation in elderly haemodialysis patients.
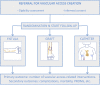
Methods and analysis: OASIS is a multicentre randomised controlled trial with an equal participant allocation in three treatment arms. Patients aged 70 years or older who are expected to initiate haemodialysis treatment in the next 6 months or who have started haemodialysis urgently with a catheter will be enrolled. To detect and exclude patients with an unusually long life expectancy, we will use a previously published mortality prediction model after external validation. Participants allocated to the usual care arm will be treated according to current guidelines on vascular access creation and will undergo fistula creation. Participants allocated to one of the two intervention arms will undergo graft placement or catheter insertion. The primary outcome is the number of access-related interventions required for each patient-year of haemodialysis treatment. We will enrol 195 patients to have sufficient statistical power to detect an absolute decrease of 0.80 interventions per year.
Ethics and dissemination: Because of clinical equipoise, we believe it is justified to randomly allocate elderly patients to the different vascular access strategies. The study was approved by an accredited medical ethics review committee. The results will be disseminated through peer-reviewed publications and will be implemented in clinical practice guidelines.
Trial registration number: NL7933.
Protocol version and date: V.5, 25 February 2021.
Keywords: adult nephrology; dialysis; end-stage renal failure; vascular surgery.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
