Different α-synuclein prion strains cause dementia with Lewy bodies and multiple system atrophy
- PMID: 35115402
- PMCID: PMC8833220
- DOI: 10.1073/pnas.2113489119
Different α-synuclein prion strains cause dementia with Lewy bodies and multiple system atrophy
Abstract
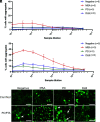
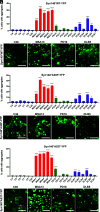
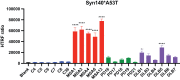
The α-synuclein protein can adopt several different conformations that cause neurodegeneration. Different α-synuclein conformers cause at least three distinct α-synucleinopathies: multiple system atrophy (MSA), dementia with Lewy bodies (DLB), and Parkinson's disease (PD). In earlier studies, we transmitted MSA to transgenic (Tg) mice and cultured HEK cells both expressing mutant α-synuclein (A53T) but not to cells expressing α-synuclein (E46K). Now, we report that DLB is caused by a strain of α-synuclein prions that is distinct from MSA. Using cultured HEK cells expressing mutant α-synuclein (E46K), we found that DLB prions could be transmitted to these HEK cells. Our results argue that a third strain of α-synuclein prions likely causes PD, but further studies are needed to identify cells and/or Tg mice that express a mutant α-synuclein protein that is permissive for PD prion replication. Our findings suggest that other α-synuclein mutants should give further insights into α-synuclein prion replication, strain formation, and disease pathogenesis, all of which are likely required to discover effective drugs for the treatment of PD as well as the other α-synucleinopathies.
Keywords: dementia with Lewy bodies; neurodegeneration; prions; strains; synucleinopathies.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: S.B.P. is a member of the Scientific Advisory Boards of ViewPoint Therapeutics and New Ventures Inc. and a member of the Supervisory Board of Priavoid, none of which have contributed financial or any other support to these studies.
Figures




References
-
- Parkinson J., An Essay on the Shaking Palsy (Sherwood, Neely, and Jones, London, 1817).
-
- Lewy F. H., “Paralysis agitans” in Pathologische Anatomie. Handbuch der Neurologie, Lewandowsky M., Ed. (Springer Verlag, Berlin, 1912), pp. 920–933.
-
- Tretiakoff C., Contribution à l’étude de l’anatomie pathologique du locus niger de Soemmering avec quelques déductions relatives à la pathogenie des troubles du tonus musculaire et de la maladie de Parkinson (University of Paris, Paris, 1919).
-
- Polymeropoulos M. H., et al. , Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047 (1997). - PubMed
-
- Spillantini M. G., et al. , α-synuclein in Lewy bodies. Nature 388, 839–840 (1997). - PubMed

