TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models
- PMID: 35115492
- PMCID: PMC8813949
- DOI: 10.1038/s41467-021-27948-4
TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models
Abstract
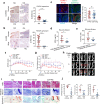
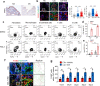
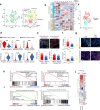
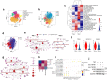
Ferroptosis is a nonapoptotic cell death process that requires cellular iron and the accumulation of lipid peroxides. In progressive rheumatoid arthritis (RA), synovial fibroblasts proliferate abnormally in the presence of reactive oxygen species (ROS) and elevated lipid oxidation. Here we show, using a collagen-induced arthritis (CIA) mouse model, that imidazole ketone erastin (IKE), a ferroptosis inducer, decreases fibroblast numbers in the synovium. Data from single-cell RNA sequencing further identify two groups of fibroblasts that have distinct susceptibility to IKE-induced ferroptosis, with the ferroptosis-resistant fibroblasts associated with an increased TNF-related transcriptome. Mechanistically, TNF signaling promotes cystine uptake and biosynthesis of glutathione (GSH) to protect fibroblasts from ferroptosis. Lastly, low dose IKE together with etanercept, a TNF antagonist, induce ferroptosis in fibroblasts and attenuate arthritis progression in the CIA model. Our results thus imply that the combination of TNF inhibitors and ferroptosis inducers may serve as a potential candidate for RA therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Comment in
-
TNF inhibition enhances depletion of synovial fibroblasts by ferroptosis.Nat Rev Rheumatol. 2022 Apr;18(4):186. doi: 10.1038/s41584-022-00769-5. Nat Rev Rheumatol. 2022. PMID: 35273386 No abstract available.
References
-
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365:2205–2219. - PubMed
-
- Giannini D, et al. One year in review 2020: pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2020;38:387–397. - PubMed
-
- McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–2337. - PubMed
-
- Silvagni E, et al. One year in review 2020: novelties in the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 2020;38:181–194. - PubMed
-
- Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389:2338–2348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

