Simple, sensitive, specific self-sampling assay secures SARS-CoV-2 antibody signals in sero-prevalence and post-vaccine studies
- PMID: 35115570
- PMCID: PMC8814240
- DOI: 10.1038/s41598-022-05640-x
Simple, sensitive, specific self-sampling assay secures SARS-CoV-2 antibody signals in sero-prevalence and post-vaccine studies
Abstract
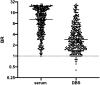
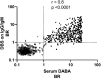
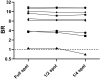
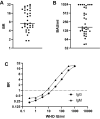
At-home sampling is key to large scale seroprevalence studies. Dried blood spot (DBS) self-sampling removes the need for medical personnel for specimen collection but facilitates specimen referral to an appropriately accredited laboratory for accurate sample analysis. To establish a highly sensitive and specific antibody assay that would facilitate self-sampling for prevalence and vaccine-response studies. Paired sera and DBS eluates collected from 439 sero-positive, 382 sero-negative individuals and DBS from 34 vaccine recipients were assayed by capture ELISAs for IgG and IgM antibody to SARS-CoV-2. IgG and IgM combined on DBS eluates achieved a diagnostic sensitivity of 97.9% (95%CI 96.6 to 99.3) and a specificity of 99.2% (95% CI 98.4 to 100) compared to serum, displaying limits of detection equivalent to 23 and 10 WHO IU/ml, respectively. A strong correlation (r = 0.81) was observed between serum and DBS reactivities. Reactivity remained stable with samples deliberately rendered inadequate, (p = 0.234) and when samples were accidentally damaged or 'invalid'. All vaccine recipients were sero-positive. This assay provides a secure method for self-sampling by DBS with a sensitivity comparable to serum. The feasibility of DBS testing in sero-prevalence studies and in monitoring post-vaccine responses was confirmed, offering a robust and reliable tool for serological monitoring at a population level.
© 2022. The Author(s).
Conflict of interest statement
The authors (CR, PC, MOM, RST) declare an in interest in the Imperial Hybrid DABA. Patent file IRN.FID4816059 and the S1 Capture Assay Patent Filing 2014047.1. The remaining authors have no conflicts of interest to declare.
Figures



References
-
- Ward, H. et al. Antibody prevalence for SARS-CoV-2 in England following first peak of the pandemic: REACT2 study in 100,000 adults. medRxiv. 10.1101/2020.08.12.20173690 (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

